Table 1. Rh-Catalyzed o-C–H Alkynylation of Ethyl Benzoate and Benzyl Methyl Ether: Optimization Conditions24.
| entry | DG | variation from the “standard conditions”a | yield (%)b |
|---|---|---|---|
| 1 | ester | none | 58–69 |
| 2 | ester | at 25 °Cc | 35 |
| 3 | ester | at 65 °Cc | 16 |
| 4 | ester | with Ag2CO3 (0.5 equiv)d | 41 |
| 5 | ester | with K2CO3 (1 equiv)d | 5 |
| 6 | ester | in dichloromethanee | 8–14 |
| 7 | ester | in toluenee | 0 |
| 8 | ester | in t-AmOHe | 0 |
| 9 | ester | in Et2Oe | 4 |
| 10 | ester | in EtOAce | 18 |
| 11 | ester | in MeOHe | 0 |
| 12 | ether | none | 0 |
| 13 | ether | at 100 °Cc | 50–64 |
| 14 | ether | without [Cp*RhCl2]2 | 0 |
| 15 | ether | without Ag2CO3 | 0 |
| 16 | ether | without LiOAc | 0 |
| 17 | ether | without AgSbF6 | 0 |
| 18 | ether | with AgOAc (1.2 equiv)f | <1.5 |
| 19 | ether | AgOAc (1 equiv) + Ag2CO3 (0.2 equiv)g | 12 |
| 20 | ether | in toluenee | 0 |
| 21 | ether | in tert-amOHe | 0 |
| 22 | ether | in 1,4-dioxanee | 0 |
| 23 | ether | with TIPS-acetyleneh | 0 |
| 24 | ether | with [Cp*IrCl2]2i | 0 |
| 25 | ether | with Pd(OAc)2i | 0 |
| 26 | ether | with [RuCl2(p-cymene)]2i | <3 |
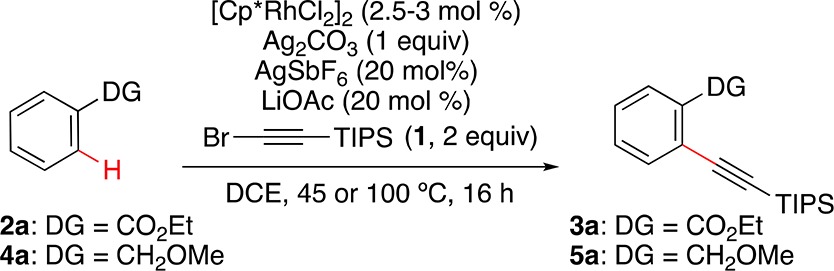
Standard reaction conditions: 2a or 4a (0.2 mmol), 1 (2 equiv), [Cp*RhCl2]2 (2.5 mol % for DG = ester, 3 mol % for DG = ether), Ag2CO3 (1 equiv), AgSbF6 (0.2 equiv), LiOAc (0.2 equiv), DCE, 16 h, 45 °C.
Yield of the monoalkynylated product determined by 1H NMR using bromomesitylene as internal standard.
Instead of 45 °C.
Instead of Ag2CO3 (1 equiv).
Instead of DCE.
Instead of Ag2CO3 and LiOAc.
Without LiOAc.
Instead of 1.
Instead of [Cp*RhCl2]2.
