A 44-year-old male patient (body mass index [BMI], 21.22 kg/m2) was admitted to our hospital with intermittent severe epigastric pain radiating to the left shoulder for 2 months. He had a history of acute pancreatitis 4 months prior when his BMI was 32.65 kg/m2. Magnetic resonance cholangiopancreatography revealed two cystic lesions, one at the pancreatic tail measuring approximately 7.5 cm × 7.0 cm × 3.5 cm and the other within the splenic capsule measuring approximately 13.0 cm × 10.0 cm × 5.5 cm [Figure 1]. The patient was diagnosed with pancreatic pseudocyst (PP) and splenic subscapular collection (SSC) complicated by moderately severe acute pancreatitis.
Figure 1.
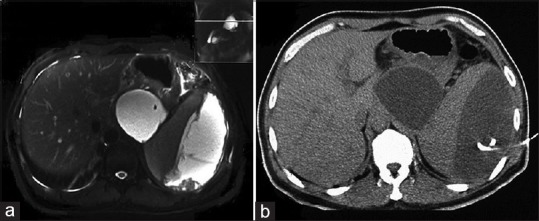
Images of the two cystic lesions. (a) Magnetic resonance cholangiopancreatography shows a splenic fluid space attached to the pancreatic tail and pancreatic pseudocyst at the pancreatic tail. (b) Computed tomography-guided percutaneous drainage of the splenic subcapsular collection
After admission, computed tomography-guided percutaneous drainage of the SSC was performed with a 10-Fr pigtail tube. The amylase level of the brown fluid was 5641 U/L. The tube was removed 8 days later when the drainage volume decreased to 10 mL/day. An EUS-guided cystogastrostomy was performed 4 days after percutaneous puncture in the upper gastric body, adjacent to the cardia. The lumen of the lumen-apposing metal stent (LAMS) showed an opening toward the esophagus and complete coverage of the gastric cardia with anchor flange, which is supposed to be in the stomach [Figure 2]. A nasogastric feeding tube was deployed instantaneously, by passing the stent, under endoscopic control [Figure 3]. The amylase level of the cyst fluid was 66,096 U/L.
Figure 2.
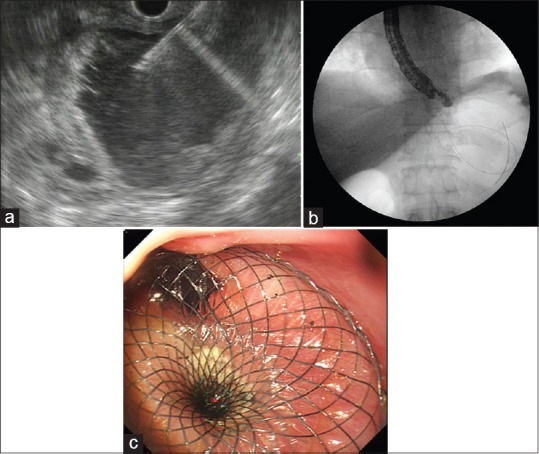
Drainage of the pancreatic pseudocyst. (a) EUS-guided puncture. (b) Fluoroscopic image immediately after deployment of lumen-apposing metal stent within the pseudocyst. (c) Endoscopic view of lumen-apposing metal stent immediately after deployment as viewed from the lower esophagus
Figure 3.
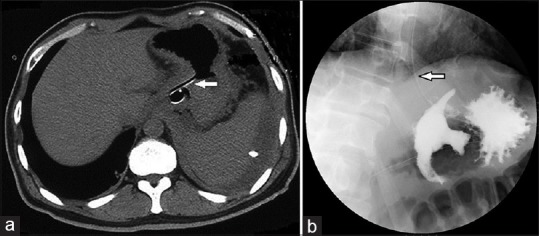
Imaging 3 days after drainage. Nasogastric feeding tube ensures adequate intake (arrowhead). (a) Computed tomography showing lumen-apposing metal stent and percutaneous drainage tube with resolution of lesions. (b) Fluoroscopy showing lumen-apposing metal stent and iodixanol flowing into the pseudocyst
By day 3, the patient reported complete resolution of pain and requested oral intake. Upper gastrointestinal contrast fluoroscopy revealed iodinated contrast media flowing into the PP cavity along the lumen of the LAMS [Figure 3]. The nasogastric tube was then used for enteral nutrition. The patient experienced rapid improvement and complete resolution of the PP upon imaging at the 3-week follow-up. The LAMS was retrieved endoscopically. An abdominal radiography was performed 60 h after removal to ensure the absence of gastric fistula [Figure 4]. Oral eating was then permitted and the patient remains asymptomatic 3 months later.
Figure 4.
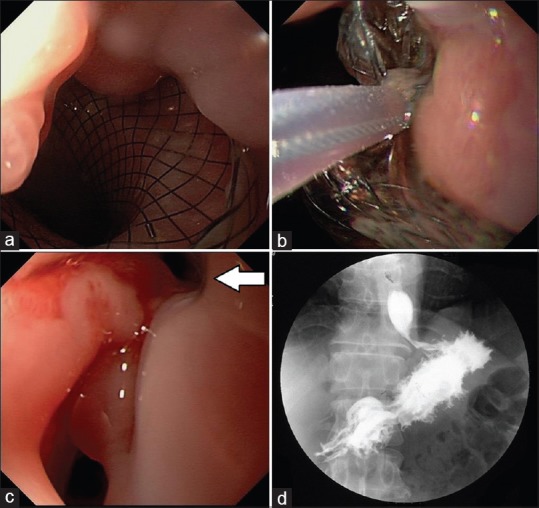
Stent removal. (a) Mucosal overgrowth at the gastric end of the stent after 27 days. (b) Safe removal using a snare. (c) Endoscopic view of fistula after removal (arrowhead). (d) Fluoroscopy confirmed healing of the gastric fistula
Splenic complications can be expected in acute pancreatitis, especially with an inflammatory process originating from the tail of the pancreas into the hilum of the spleen, which likely occurred in our case. Percutaneous drainage is the recommended treatment option for these complications.[1,2] EUS-guided cystogastrostomy of a PP is a well-established procedure.[3,4,5] Recently, the introduction of the novel LAMS has made transmural intervention more effective.[6,7,8] In our case, the cystogastrostomy process was imperfect because the cardia was blocked because of the upper puncture position. Fortunately, the large stent diameter allowed rapid adequate drainage and the nutrition tube was just placed for 27 days. We recommend choosing the lower part of the stomach as the puncture point, or designing a shorter LAMS, to avoid this complication.
Acute pancreatitis complicated simultaneously by PP and SSC is not uncommon. We treated both conditions during the initial hospitalization. Cardia occlusion by LAMS during the EUS-guided procedure retarded oral administration; we are the first to report this complication associated with LAMS.[9,10] This was successfully solved by placement of a nasogastric feeding tube.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that his name and initial will not be published and due efforts will be made to conceal his identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Malka D, Hammel P, Lévy P, et al. Splenic complications in chronic pancreatitis: Prevalence and risk factors in a medical-surgical series of 500 patients. Br J Surg. 1998;85:1645–9. doi: 10.1046/j.1365-2168.1998.00952.x. [DOI] [PubMed] [Google Scholar]
- 2.Tseng CW, Chen CC, Chiang JH, et al. Percutaneous drainage of large subcapsular hematoma of the spleen complicating acute pancreatitis. J Chin Med Assoc. 2008;71:92–5. doi: 10.1016/S1726-4901(08)70081-9. [DOI] [PubMed] [Google Scholar]
- 3.Wiersema MJ. Endosonography-guided cystoduodenostomy with a therapeutic ultrasound endoscope. Gastrointest Endosc. 1996;44:614–7. doi: 10.1016/s0016-5107(96)70022-6. [DOI] [PubMed] [Google Scholar]
- 4.Vilmann P, Hancke S, Pless T, et al. One-step endosonography-guided drainage of a pancreatic pseudocyst: A new technique of stent delivery through the echo endoscope. Endoscopy. 1998;30:730–3. doi: 10.1055/s-2007-1001399. [DOI] [PubMed] [Google Scholar]
- 5.Lopes CV, Pesenti C, Bories E, et al. Endoscopic-ultrasound-guided endoscopic transmural drainage of pancreatic pseudocysts and abscesses. Scand J Gastroenterol. 2007;42:524–9. doi: 10.1080/00365520601065093. [DOI] [PubMed] [Google Scholar]
- 6.Talreja JP, Shami VM, Ku J, et al. Transenteric drainage of pancreatic-fluid collections with fully covered self-expanding metallic stents (with video) Gastrointest Endosc. 2008;68:1199–203. doi: 10.1016/j.gie.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues-Pinto E, Baron TH. Evaluation of the AXIOS stent for the treatment of pancreatic fluid collections. Expert Rev Med Devices. 2016;13:793–805. doi: 10.1080/17434440.2016.1222898. [DOI] [PubMed] [Google Scholar]
- 8.Zhu H, Lin H, Jin Z, et al. Re-evaluation of the role of lumen-apposing metal stents (LAMS) for pancreatic fluid collection drainage. Gut. 2017;66:2192. doi: 10.1136/gutjnl-2017-313949. [DOI] [PubMed] [Google Scholar]
- 9.Shah RJ, Shah JN, Waxman I, et al. Safety and efficacy of endoscopic ultrasound-guided drainage of pancreatic fluid collections with lumen-apposing covered self-expanding metal stents. Clin Gastroenterol Hepatol. 2015;13:747–52. doi: 10.1016/j.cgh.2014.09.047. [DOI] [PubMed] [Google Scholar]
- 10.Rinninella E, Kunda R, Dollhopf M, et al. EUS-guided drainage of pancreatic fluid collections using a novel lumen-apposing metal stent on an electrocautery-enhanced delivery system: A large retrospective study (with video) Gastrointest Endosc. 2015;82:1039–46. doi: 10.1016/j.gie.2015.04.006. [DOI] [PubMed] [Google Scholar]


