Abstract
Background
Negative neurocognitive bias is a core feature of depression that is reversed by antidepressant drug treatment. However, it is unclear whether modulation of neurocognitive bias is a common mechanism of distinct biological treatments. This randomized controlled functional magnetic resonance imaging study explored the effects of a single electroconvulsive therapy session on self-referent emotional processing.
Methods
Twenty-nine patients with treatment-resistant major depressive disorder were randomized to one active or sham electroconvulsive therapy session at the beginning of their electroconvulsive therapy course in a double-blind, between-groups design. The following day, patients were given a self-referential emotional word categorization test and a free recall test. This was followed by an incidental word recognition task during whole-brain functional magnetic resonance imaging at 3T. Mood was assessed at baseline, on the functional magnetic resonance imaging day, and after 6 electroconvulsive therapy sessions. Data were complete and analyzed for 25 patients (electroconvulsive therapy: n = 14, sham: n = 11). The functional magnetic resonance imaging data were analyzed using the FMRIB Software Library randomize algorithm, and the Threshold-Free Cluster Enhancement method was used to identify significant clusters (corrected at P < .05).
Results
A single electroconvulsive therapy session had no effect on hippocampal activity during retrieval of emotional words. However, electroconvulsive therapy reduced the retrieval-specific neural response for positive words in the left frontopolar cortex. This effect occurred in the absence of differences between groups in behavioral performance or mood symptoms.
Conclusions
The observed effect of electroconvulsive therapy on prefrontal response may reflect early facilitation of memory for positive self-referent information, which could contribute to improvements in depressive symptoms including feelings of self-worth with repeated treatments.
Keywords: ECT, fMRI, depression, self-referent memory, emotional bias
Significance Statement
Negative neurocognitive bias is a core feature of depression that is reversed by antidepressant drug treatment. This randomized, double-blind, sham-controlled fMRI study shows for the first time that a single ECT session influences neuronal response in the prefrontal cortex during retrieval of emotional self-referent words. The effect may reflect increased memory efficiency for positive self-referent information and highlights modulation of negative neurocognitive bias as a putative common mechanism of distinct biological treatments for depression.
Introduction
According to the cognitive theory of depression, negative bias in the cognitive processing of emotional information is a central component in the development and maintenance of depression (Beck and Alford, 2014) and increases the risk of depressive relapse (Bouhuys et al., 1999; Mathews and MacLeod, 2005). This negative bias is particularly evident in the processing of personally relevant information such as self-referent emotional words (Miskowiak and Carvalho, 2014). Specifically, patients with major depressive disorder (MDD) display slowed responses to and impaired recall of positive self-referent words and increased memory for negative compared with positive and neutral words during acute episodes (Bradley and Mathews, 1983; Watkins et al., 1992; Neshat-Doost et al., 1998; Dozois and Dobson, 2001; Harmer et al., 2009). Functional magnetic resonance imaging (fMRI) studies show that this negative bias is accompanied by hyper-activity in the medial prefrontal cortex, rostral anterior cingulate cortex, and insula during encoding of negative self-referent words and hippocampal hypo-activity during encoding of positive words (van Tol et al., 2012; Yoshimura et al., 2010).
Studies of whether negative self-referent memory bias persists after clinical remission have produced equivocal results. In one study, remitted patients endorsed and recalled more negative self-referent words compared with healthy controls (Romero et al., 2014). However, other studies showed either no negative bias for self-referent words (Dobson and Shaw, 1987) or a necessity for induction of sad mood state to detect reduced recall of positive self-referent words in remitted patients (Ramel et al., 2007). We recently found that monozygotic and dizygotic twins at familial risk of depression display subtle negative recall bias for self-referent emotional words (more false recollections of negative words) than low-risk twins (unpublished observations). In contrast, other studies observed no negative self-referent memory bias in unaffected individuals at familial risk of depression (Mannie et al., 2007; Wolfensberger et al., 2008). Nevertheless, aberrant neural response in the inferior frontal gyrus was detected in these at-risk individuals during encoding and recognition of negative words (Wolfensberger et al., 2008). The consistent evidence for negative self-referent memory bias in symptomatic patients and less consistent findings in remitted patients and at-risk individuals point to negative memory bias as a state marker of depression that is at least partially resolved after successful treatment.
Indeed, modulation of negative bias in the processing and recall of self-referent information may play a key role in the clinical efficacy of antidepressant drugs (Harmer and Cowen, 2013). Specifically, a single dose of the selective noradrenaline reuptake inhibitor reboxetine reverses negative bias in the categorization and recall of self-referent words in depressed patients prior to observable changes in mood symptoms (Harmer et al., 2009). Similarly, a single dose of reboxetine facilitated recognition of positive vs negative self-referent words in healthy individuals, which was accompanied by reduced neuronal activation in a fronto-parietal network during recognition of positive target words vs matched distractors (Miskowiak et al., 2007). Finally, short-term treatment with citalopram reduced ventromedial prefrontal response to negative self-referential words in a population of individuals at risk for depression, indicated by high neuroticism (Di Simplicio et al., 2012). Interestingly, a case report of vagus nerve stimulation in a depressed patient showed treatment-related modulation of ventral prefrontal cortex response during encoding of negative self-referent words, which was accompanied by reduced recognition of negative (but not positive or neutral) words (Critchley et al., 2007). Aside from this single case report study, it is unknown whether other biological treatments for depression produce similar effects on self-referent emotional memory that could be mechanistically important for their therapeutic effects.
Electroconvulsive therapy (ECT) is the most effective and fast-acting treatment for severe depression (The UK ECT Review Group, 2003). Putative neurobiological mechanisms of ECT are increase in brain-derived neurotrophic factor, neurogenesis (Madsen et al., 2000; Altar et al., 2004; Bolwig and Madsen, 2007), and hippocampal volume (Jorgensen et al., 2016). However, it is unclear whether ECT modulates neural and behavioral response to emotional information in the same manner as antidepressant pharmacotherapy. We recently demonstrated in a randomized, sham-controlled, double-blind study that a single ECT session modulates the neural (but not behavioral) response to emotional faces in patients with MDD (Miskowiak et al., 2017). The present study of the same patient cohort aimed to investigate whether a single ECT vs sham session modulates neuronal and cognitive measures of memory for self-referent emotional information in patients with MDD. We hypothesized that ECT would facilitate the retrieval of positive vs negative self-referent words, as indicated by: (1) reduced neural response in frontal and parietal regions during recognition of positive words vs matched distractor words (i.e., greater efficiency of memory retrieval for positive words) (primary outcome), as well as (2) increased retrieval-specific hippocampal (but not amygdala) (Miskowiak et al., 2007) response to positive than negative words, and (3) improved recall of positive vs negative words (secondary outcomes). These effects were expected to occur prior to any differential change between ECT and sham groups in depressive symptoms or subjective state.
Methods
Study Design and Patients
Patients scheduled for ECT were recruited from Psychiatric Centre Copenhagen (Rigshospitalet and Bispebjerg) from November 2009 to July 2015. Personnel at the respective departments carried out eligibility assessments in terms of a diagnosis of depression and established degree of depressive symptom severity using the Hamilton Depression Rating Scale 17-items (HDRS-17) (Hamilton, 1960). The inclusion criterion was current moderate to severe depression, defined by a HDRS-score ≥18. Exclusion criteria were current substance- or alcohol misuse disorders, neurological disorders, bipolar depression, schizotypal disorder or schizophrenia, pregnancy, major somatic illness contraindicating ECT, and (for fMRI scanning) having a pacemaker or other metal implants in the body. The study was approved by the local ethics committee (H-3-2009-074) and the Danish Data Protection Agency (2009-41-3676) and was carried out in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Randomization and Masking
Block randomization was performed by Pharma Consulting Group (Uppsala, Sweden) with stratification for age (< or≥45 years) and gender. Pharma Consulting Group created a randomization list and envelopes with information about treatment allocation (ECT/sham) for each patient ID number. Along with date of birth and gender, treatment allocation was assigned to each patient upon inclusion. Numbering was consecutive, and the ID number was recorded in each patient’s individual file. The randomization list was kept in a locked cabinet in the ECT room, to which only personnel involved in ECT/sham treatment had access. Patients, personnel, and outcome assessors were blinded to group assignment and blinding was maintained throughout the study, data management, and outcome assessment.
Procedure
Baseline mood ratings were performed during the screening interview (1–4 days prior to the first ECT or sham session). Patients were then randomized to either a single ECT or sham treatment at the beginning of their ECT series. For this first ECT/sham treatment, electrodes were placed bilaterally and patients were put under full anesthesia using thiopental and given succinylcholine for muscle relaxation. After this, an envelope with information about the particular patient’s treatment allocation was opened and either active or sham ECT was administered accordingly with a Thymatron ECT Machine. The ECT pulse width range was 0.5 to 1.0 milliseconds and the initial dose based on the patient’s age was used (charge [per cent of 500 millicoulomb] = 50% of the age).
One day after the initial ECT/sham session (day 1), patients attended a test session of approximately 2 hours at the Danish Research Center for Magnetic Resonance. During this time, they performed a set of computerized emotional processing tests from the Emotional Test Battery (Oxford, P1 Vital) before and during fMRI and a nonemotional verbal memory test after the scan. For clarity, this report includes only the results of the self-referent emotional word categorization, recall, and recognition tests from the Emotional Test Battery and the nonemotional verbal memory test. Mood symptoms and subjective state were assessed using the HDRS-17 and relevant questionnaires. From day 3 and onwards, all patients received active ECT treatment according to the standard protocol of the Capital Region (3 times/wk). For these subsequent ECT sessions, a titration based on seizure quality (configuration and length of the EEG seizure that should exceed 25 seconds) was applied with 50% dosage increase in case of threshold seizure. Mood symptoms were rated with the HDRS-17 after 6 (active) ECT sessions. End of treatment was decided by the treating psychiatrist.
Neurocognitive Tests
Patients were given a self-referent emotional categorization task followed by a free recall tests before entering the scanner (Anderson, 1968). A total of 90 unambiguously negative and positive personality trait words from the Anderson’s list of personality trait words (Anderson, 1968), matched for length, frequency, and meaningfulness, were displayed on the screen of a laptop computer. The words were presented one at a time for 500 milliseconds in a random order with each word occurring only once. Patients were instructed to press 1 of the 2 keys on a keyboard as quickly and accurately as possible to categorize the words as likeable or unlikeable in a self-referential manner. Specifically, they were asked to imagine that they overheard someone talking about them and decide if they would be happy or sad if these words were used to describe them. Accuracy and response time were recorded using Superlab software. Fifteen minutes later, patients were given a free recall test, which involved recall of as many positive and negative words from the task as possible for a maximum period of 5 minutes. The total numbers of correctly recalled positive and negative words and of false recollections of positive and negative words (memory intrusions) were recorded.
Inside the scanner, patients were given an incidental emotional recognition task. A total of 180 words was projected onto an opaque screen viewed by patients through angled mirrors. Of these, 90 words had been presented in the categorization test (old words) and 90 were new matched distractors (45 positive and 45 negative) from the Anderson’s list (new words). Patients were instructed to indicate as quickly and accurately as possible whether the words displayed were old or new by pressing corresponding keys on a key pad. The paradigm had an event-related design with each trial consisting of a fixation cross shown for 500 ms followed by a personality trait words displayed for 500 ms. Words were presented in a random order with an inter-trial interval between 4000 and 9000 milliseconds, resulting in a total task time of 12 minutes. Accuracy and response times for correctly recognized words and misclassifications were recorded with e-prime software version 1.2 (Psychology Software Tools Inv.). In addition, a visual stimulation control task (with minimal cognitive demands) was implemented to investigate whether any potential effects of ECT on recognition-related neural activity were confounded by global changes in cerebral blood flow.
A flashing checkerboard (frequency of 8 Hz) was presented in blocks of 14 seconds interleaved by a 14-second fixation cross for a total of 6 cycles. Patients were instructed to keep their eyes open during this time.
After the scan, patients were given the Rey Auditory Verbal Learning Test (RAVLT) (Schmidt, 1996) for assessment of nonemotional verbal memory. Total recall across trials I to V, immediate recall following interference, delayed free recall (after 30 minutes), and recognition were recorded.
Mood and Subjective State
Depressive symptoms were assessed with the HDRS-17 and the Beck Depression Inventory (BDI) (Beck et al., 1961). Anxiety and subjective state were assessed at the time of fMRI scanning with the State Trait Anxiety Inventory (STAI) (Spielberger, 1983) and visual analogues scales (VAS) for happiness, sadness, alertness, anxiety, dizziness and nausea.
MRI
MRI data were collected with a 3 T Siemens Trio MR scanner using an 8-channel head array coil. Blood oxygen-level dependent (BOLD)-sensitive fMRI used a T2*-weighted gradient echo spiral echo-planar imaging sequence with an echo time of 30 milliseconds, repetition time of 2.49 ms, and a low flip angle of 20° to minimize physiological noise (Gonzalez-Castillo et al., 2011). A total of 128 brain volumes were acquired in a single fMRI session, each consisting of 42 slices with a slice thickness of 3 mm and a field of view of 192×192 mm using a 64×64 grid. High-resolution 3D structural T1-weighted spin echo images were obtained after the first session of BOLD fMRI (TI=800, echo time =3.93, repetition time =1540 ms, flip angle 9°; 256×256 field of view; 192 slices).
Statistical Analysis of Behavioral Data and Mood Symptoms
Behavioral data from the emotional categorization task (speed and accuracy), free recall (total recall and memory intrusions), and recognition (speed, accuracy and response bias) were analyzed using repeated measures ANCOVA with valence (positive, negative) as the within-subjects factor and treatment (ECT, sham) as the between-subjects factor and adjustment for potential differences in baseline characteristics. For the emotional recognition task, we used signal detection theory to obtain a measure of memory accuracy corrected for the patients’ response tendency. The proportion of correctly recognized words and of falsely recognized words constitute the parametric sensitivity measure: d’=(number of hits+0.5/number of targets+1) - (number of false alarms +0.5/number of distractors+1). In addition, response bias was computed according to false-alarm scores: (number of false alarms+0.5/number of distractors +1)/(1 - Pr). This measure reflects the tendency of participants, when uncertain about the category to which a word should belong, to categorize the word as old rather than new. We applied Sidak correction for the number of statistical comparisons across the behavioral tests (n=6), leading to an adjusted threshold for significance of P=.009. Significant interactions were followed up by independent samples t tests for normally distributed data or Mann-Whitney U tests for nonnormally distributed data. Performance on the RAVLT, mood symptoms (HDRS-17 and BDI scores), and anxiety (STAI scores) were analyzed with independent samples t tests. Finally, VAS ratings of subjective state were examined with repeated-measures ANOVA. Analyses of behavioral data, mood, and subjective state were performed with the Statistical Package for Social Sciences (SPSS) (version 22.0) (IBM Corporation).
fMRI Data Analysis
Functional MRI data processing was performed with the FMRI Expert Analysis Tool (version 6.00) part of FMRIB’s Software Library (www.fmrib.ox.ac.uk/fsl). Preprocessing involved image realignment, non-brain removal, spatial normalization to an MNI (Montreal Neurologic Institute) template, and spatially smoothing (Gaussian kernel, 5 mm full-width-half-maximum). The time series in each session were high pass-filtered (to max 0.008 Hz).
Events of interest were defined according to whether or not the word was presented in the previous categorization task (old vs new), whether the word was positive or negative, and whether the word was correctly identified as old (hit) or new (correct rejection). At the individual subject level, we therefore set up a general linear model (GLM) that included 8 event types: (1) positive/hit, (2) positive/correct rejection, (3) negative/hit, (4) negative/correct rejection, (5) incorrect positive/hit (i.e., incorrect identification of new positive words as “old”), (6) incorrect negative/hit, (7) incorrect positive/rejection (incorrect identification of old positive words as “new”), and (8) incorrect negative/rejection. The events were convolved with a canonical hemodynamic response function (Boynton et al., 1996), and the GLM model included local autocorrelation correction (Woolrich et al., 2001).
To examine our hypothesis that ECT would influence neural activity in areas involved in retrieval-specific processes for positive and negative words rather than valence per se, we defined 3 contrasts of interest: (1) positive/hits vs positive/correct rejections and (2) negative/hits vs negative/correct rejections (i.e., treatment by task interactions). Finally, to investigate the effects of ECT on neural activity on valence-specific memory processes, we contrasted (3) negative/hits with positive/hits. At the group level, data for the 3 contrasts of interest were included in separate GLM models estimated using nonparametric permutation-based inference (n=5000) using the randomize algorithm implemented in FMRIB’s Software Library (Winkler et al., 2014).
We first investigated the primary hypothesis that ECT would modulate the response in fronto-parietal regions during retrieval of positive words, specifically. We therefore defined a volume of interest (VOI) mask on a standard MNI template that included the bilateral prefrontal and parietal cortical maps provided by the Harvard-Oxford cortical structural Atlas thresholded at 5%. The prefrontal cortex included the cortical regions anterior to the precentral sulcus, that is, superior, middle, and inferior frontal gyri; the frontal medial cortex and subgenual cortices; and the frontal poles. The parietal regions included the precuneus and superior parietal lobe. We then investigated the secondary hypothesis that ECT would increase hippocampal response during retrieval of positive relative to negative words. Bilateral hippocampal formations were therefore defined using maps included in the Harvard-Oxford Subcortical Structural Atlas and thresholded at 5%. The statistical inference at group level was restricted within the defined VOIs. We further carried out a whole-brain analysis to explore possible effects of ECT in the 3 contrasts of interest in other brain regions.
Significant clusters were identified using the Threshold-Free Cluster Enhancement method at corrected P<.05. Upon significant findings, we first conducted a posthoc analysis to explore whether the effects of the ECT intervention were dependent on the individual task performance. To control for the effect of performance, we set up an analogue GLM model and added an accuracy covariate calculated as the individual average correct recognitions across positive and negative old and new words. Secondly, we explored whether the mean percent signal change in the region responding to the ECT intervention was correlated with the speed or accuracy in the recognition for positive words, or change in mood symptoms (HRDS-17 scores) from baseline to the sixth ECT session. Patients with very low recognition accuracy, defined a priori as ≥2 SD below from the group mean were excluded from fMRI analyses.
Results
Participant Characteristics and Mood
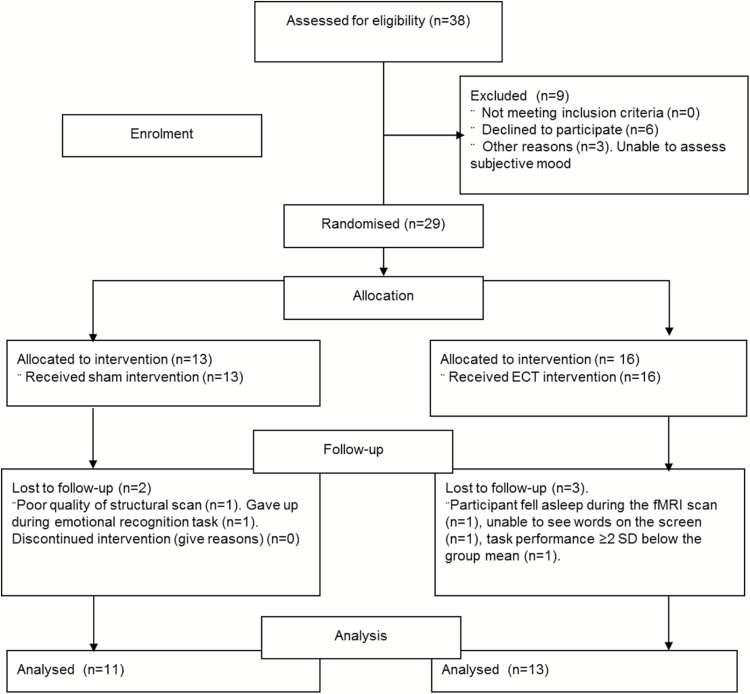
Of the 29 patients randomized to either ECT or sham treatment (ECT: n=16, sham: n=13), one participant (ECT) fell asleep during the fMRI scan, the image quality for the structural scan was inadequate for another participant (sham), and one patient (ECT) had a task performance ≥2 SD below the group mean and was therefore excluded from the fMRI analysis. Two patients failed to complete the emotional recognition task (ECT: n=1, sham: n=1). Finally, data for one patient (ECT) from the control visual stimulation paradigm was lost due to technical difficulties, but the patient’s fMRI data from the recognition paradigm were intact and included in the analyses. Data were therefore analyzed for 25 patients (ECT: n=14, sham: n=11) (see Figure 1 for CONSORT flow diagram). Self-reported mood and subjective state ratings were missing for 5 patients (for details, see Table 1), RAVLT data were missing for one patient (sham), data from the emotional categorization task were lost for one participant (sham), and data from the emotional recognition were missing for one participant (sham) (see details in Table 3).
Figure 1.
CONSORT flow diagram.
Table 1.
Demographic and Clinical Characteristics at Baseline
| Sham (n=11) | ECT (n=14) | P value | |
|---|---|---|---|
| Gender, n/% males | 7/63 | 8/53 | .7 |
| Age, median (IQR) | 37 (32) | 40 (29) | .9 |
| Age at onset, median (IQR) | 22 (24) | 18 (30) | .5 |
| First hospitalization, median (IQR) | 24 (30) | 39 (30) | .9 |
| Number of episodes, median (IQR) | 5 (8) | 9 (20) | .6 |
| Education (yrs.), mean (SD) | 16 (2) | 14 (2) | .04 |
| STAI-trait, mean (SD)a | 63 (9) | 52 (13) | .06 |
| Medicationsb | |||
| Antidepressants, n (%) | 8 (73) | 11 (85) | .5 |
| Antipsychotics, n (%) | 4 (36) | 5 (39) | .9 |
| Anticonvulsants, n (%) | 1 (9) | 1 (8) | .9 |
| Benzodiazepines, n (%) | 4 (36) | 6 (46) | .6 |
| Lithium, n (%) | 1 (9) | 0 (0) | .3 |
| Thyidea, n (%) | 0 (0) | 1 (8) | .3 |
| Combined prescriptions, n | 1.6 | 1.8 | .6 |
| Baseline | |||
| HDRS-17, mean (SD) | 28 (6) | 26 (5) | .6 |
| BDI, median (IQR)c | 25 (20) | 28 (10) | .5 |
| Day 1 | |||
| HDRS-17, mean (SD) | 26 (7) | 23 (5) | .2 |
| BDI, median (IQR)d | 17 (17) | 25 (10) | .2 |
| STAI-state, median (IQR)e | 59 (23) | 51 (21) | .7 |
| VASf | .6 | ||
| Happiness, median (IQR) | 1 (3) | 2 (4) | - |
| Sadness, mean (SD) | 7 (2) | 7 (3) | - |
| Alertness, median (IQR) | 4 (5) | 5 (6) | - |
| Anxiety, mean (SD) | 5 (3) | 4 (3) | - |
| Dizziness, median (IQR) | 2 (4) | 0 (2) | - |
| Nausea, median (IQR) | 1 (3) | 0 (1) | - |
Abbreviations: IQP, interquartile range; SD, standard deviation; STAI, State-Trait Anxiety Inventory; Yrs, years.
Table 3.
Overview of the Behavioral Data from the Categorization, Recall, and Recognition Trials
| Task and performance measure | Sham (n=11) | ECT (n=14) | Analysis | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Task [task by groupa] |
||
| P | df | |||
| Emotional categorization | ||||
| Accuracy (% correct responses) | ||||
| Positive words | 90 (8) | 89 (16) | .03 | 1,22 |
| Negative words | 94 (5) | 93 (6) | [.5] | 1,22 |
| Response times (ms) | ||||
| Positive words | 1 161 (376) | 1 007 (328) | .6 | 1,22 |
| Negative words | 1 270 (413) | 1 077 (359) | [.5] | 1,22 |
| Emotional recallb | ||||
| Positive recalled | 3 (1) | 3 (3) | .2 | 1,21 |
| Negative recalled | 3 (2) | 2 (3) | [.2] | 1,21 |
| Positive invented | 2 (2) | 2 (2) | .06 | 1,21 |
| Negative invented | 1 (1) | 1 (2) | [.5] | 1,21 |
| Emotional recognitionc | ||||
| Accuracy (percent correct responses) | ||||
| Positive/hits | 72 (18) | 68 (8) | .01 | 1,22 |
| Negative/hits | 61 (20) | 63 (11) | [.3] | 1,22 |
| Response times (ms) | ||||
| Correct trials | ||||
| Positive/hits | 1 187 (450) | 1 328 (296) | .02 | 1,22 |
| Negative/hits | 1 274 (449) | 1 340 (315) | [.06] | 1,22 |
| Positive/correct rejections | 1 347 (385) | 1 205 (426) | ||
| Negative/correct rejections | 1 331 (387) | 1 193 (443) | ||
| Incorrect trialsd | ||||
| Incorrect positive/ rejection | 1 182 (279) | 1 179 (297) | ||
| Incorrect negative/ rejection | 1 085 (211) | 1 212 (299) | ||
| Incorrect positive/hit | 1 007 (216) | 1 202 (287) | ||
| Incorrect negative/hit | 1 187 (388) | 1 219 (341) | ||
| Discrimination accuracy | ||||
| Positive words | 0.2 (0.2) | 0.1 (0.3) | .4 | 1,22 |
| Negative words | 0.2 (0.2) | 0.1 (0.2) | [.9] | 1,22 |
| Response bias | ||||
| Positive words | 0.1 (0.2) | 0.0 (0.2) | .1 | 1,22 |
| Negative words | 0.0 (0.2) | -0.1 (0.2) | [.1] | 1,22 |
aAdjusted for years of education.
bData missing for 1 participant (sham).
cAnalyses performed n=24 patients.
dData missing for 3 participants (sham: n=1, ECT: n=2).
The ECT and sham groups were comparable for gender composition, age, indexes of illness chronicity, and medication status (P≥.3). However, ECT-treated patients had somewhat shorter education than patients allocated to sham treatment (mean ± SD: ECT: 14±2; sham: 16±2; t=2.2, df =23, P=.04) (Table 1). There were no differences between the 2 groups in depressive symptom severity at baseline or on the day of the scan (P≥.2). There were also no differences between the 2 groups in subjective state or state anxiety on the day of the scan (P≥0.6). There was a nonsignificant trend towards lower trait anxiety in ECT-treated vs sham-treated patients (P=.06). As expected, all patients displayed substantial reduction in depressive symptom severity from baseline to the assessment following 6 active ECT sessions (mean score±SD after 6 ECT sessions: HDRS-17: 15±7; BDI: 14±7, P<.01). Of the 25 included patients, 11 (44%) showed treatment response (i.e., a ≥50% symptom reduction from baseline), with 5 (20%) achieving clinical remission (i.e., HDRS-17≤7) after 6 active ECTs, which is somewhat lower than the response and remission rates after complete ECT series in unipolar depression (Dierckx et al., 2012).
Behavioral Data
Across the entire cohort, patients showed greater accuracy during categorization of negative vs positive words (F(1,22)=5.2, P=.03) in the absence of differences in speed (P≥.6) (Table 3). However, accuracy and speed in the categorization of positive vs negative words did not differ between the ECT and sham groups (P≥0.5). In the free recall test, patients generally recalled an equal number of positive vs negative words, and this did not differ between the 2 treatment groups (P≥0.2). There was a trend towards more positive than negative false recollections in general (F(1,21) = 3.9, P=.06), but this did not differ between the groups (P≥0.5).
Across all patients, there was greater recognition accuracy for positive vs negative words (F(1,22)=7.4, P=.01; posthoc t test: t=2.6, df=23, P=.02), but there was no difference between the groups (P≥.03). Analysis of recognition speed including all patients demonstrated faster response times for positive vs negative words (F(1,22)=6.5, P=.02; posthoc t test: t=-2.3, df=23, P=.03). At the unadjusted statistical threshold for significance, there was a trend towards an interaction between group and speed during emotional word recognition indicating faster response times to both positive and negative words in the sham vs ECT groups (F(1,22)=3.8, P=.06; t tests nonsignificant; P≥.4). However, given our a priori Sidak correction for multiple testing (adjusted threshold: P<.009), this finding rendered nonsignificant. Discrimination accuracy (d-prime) was unaffected by valence and revealed no differences between ECT and sham groups (P≥.2). Response bias was generally higher for positive vs negatives words across the entire cohort (F(1,22)=14.4, P<.01; posthoc Wilcoxon Signed Ranks: Z=-3.0, P<.01) but showed no difference between the 2 groups (P≥.3).
Finally, there were no differences between the treatment groups in nonemotional memory as reflected by RAVLT total recall, recall following a distractor list, delayed recall, and recognition (all P≥.7).
fMRI Results
Effects of ECT vs Sham Treatment
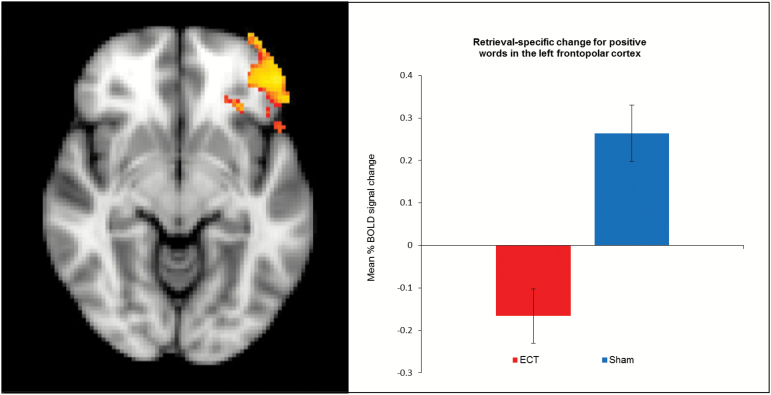
A single ECT session reduced the retrieval-specific neural response to positive words compared with sham in the left frontopolar cortex within the defined VOI (for peak cluster activation, see Table 2; for visualization of the frontopolar cluster, see Figure 2). In contrast with our hypothesis, no effects of ECT vs sham treatment were observed in retrieval-specific hippocampal activity for positive or negative words or in valence-specific activity to old negative vs positive words. Further, no differences were observed between the treatment groups in retrieval-specific response to negative words or in valence-specific activity to old positive vs old negative words. Exploratory whole brain analyses also revealed no effect of ECT vs sham in other brain regions.
Table 2.
Peak Cluster Activations for Main Effects and Between-Group Differences in Brain Regions during the Recognition Trial
| P value | Number of voxels | MNI | |||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Positive/hits vs positive/correct rejections | |||||
| Sham>ECT | |||||
| Left middle frontal gyrus (BA 10) | <.01 | 1 065 | -40 | 58 | 0 |
| Left inferior frontal gyrus (BA 45) | .01 | 25 | -58 | 22 | 6 |
| Main effect of task | |||||
| Right precuneus (BA 7)a | <.01 | 797 | 4 | -64 | 44 |
| Left superior parietal lobe (BA 7) | .03 | 43 | -22 | -76 | 52 |
| Left cuneus (BA 18) | .04 | 39 | -10 | -90 | 14 |
| Left superior occipital gyrus (BA 18) | .04 | 19 | -20 | -86 | 20 |
| All correct hits (old target words) >baseline | |||||
| Left inferior parietal lobe (BA 7/40) | .015 | 96 | -40 | -32 | 44 |
| Right inferior occipital gyrus (BA 18) | <.01 | 667 | 24 | -86 | -10 |
| Left middle occipital gyrus (BA 18) | <.01 | 239 | -18 | -92 | 4 |
| Left inferior occipital gyrus (BA 19) | .01 | 115 | -44 | -70 | -8 |
| Left fusiform gyrus (BA 19) | .04 | 28 | -28 | -68 | -16 |
| Right fusiform gyrus (BA 37) | .03 | 28 | 30 | -54 | -20 |
Abbreviations: BA,Broadmann area; MNI, Montreal Neurological Institute; PFC, prefrontal cortex; PPC, prefrontal parietal cortex; VOI, volume of Interest.
aWithin the PFC+PPC VOI analysis.
Figure 2.
Brain images: retrieval-specific response in the left frontopolar cortex for positive words (old positive minus new positive words) in electroconvulsive therapy (ECT) and sham groups. Chart: mean percent signal change in the left frontopolar cortex in the ECT and sham groups. The error bars represent the SEM.
The visual stimulation paradigm revealed no differential BOLD response to photic stimulation in the occipital cortex between ECT and sham groups (P=.8).
Task-Related Activations Across All Participants
A broad distributed neural network including several bilateral prefrontal and parietal regions was activated during recognition of old (positive and negative) words vs matched distractor words across all patients (for peak cluster activations, see Table 2). Further, contrasting old positive target words with matched distractors revealed significant retrieval-specific activity in a distributed occipito-parietal network (peak cluster activations in Table 2). However, the hippocampi were not significantly activated during retrieval of old positive or negative words vs new distractor words (retrieval-specific activity) and showed no differential activation to negative vs positive words across the entire cohort.
Exploratory Correlation Analyses
Exploratory postdoc correlation analyses showed no correlation between retrieval-specific left frontopolar activity for positive words and recognition speed for positive words (P≥.2). There was a trend toward a correlation between higher frontopolar activity and greater accuracy across all participants (P=.08), suggesting that the region may play a role in retrieval success. Across the entire cohort, there was no association between retrieval-specific frontopolar activity for positive words and patients’ mood improvement from baseline to their sixth ECT session (P≥.7).
Discussion
This is the first randomized sham-controlled fMRI-study of the effects of a single ECT session on self-referent emotional memory in depression. We found that a single ECT session reduced the neural activity in the left frontopolar cortex during retrieval of positive self-referent words. The effect occurred in the absence of treatment effects on memory-relevant hippocampal response. There were no differences between groups in performance on the self-referent emotional or nonemotional memory tests or in mood symptoms. As expected, all patients displayed improved depressive symptom severity following 6 active ECT sessions, but this was not predicted by the early modulatory effects of ECT on memory-related neural activity.
The reduced retrieval-specific neural activity in the frontopolar cortex for positive words in ECT-treated patients is remarkably similar to our previous finding that a single dose of reboxetine decreases retrieval-specific fronto-parietal activity for positive words compared with placebo in healthy volunteers (Miskowiak et al., 2007). While a single ECT session did not influence memory performance in our severely depressed patients, the effect of reboxetine on neural activity in healthy volunteers was accompanied by increased speed to recognize positive vs negative words (Miskowiak et al., 2007). It is possible that this discrepancy in behavioral findings reflects lower sensitivity of treatment effects on behavioral measures in this depressed sample due to confounding effects of medication and depressive symptoms (Miskowiak et al., 2007). Modulation of key neural circuitries may thus be a more sensitive marker of treatment efficacy than change in overt behavioral measures. Given this, it is conceivable that the observed effects of ECT on frontopolar activity indicates lesser need for prefrontal resources to retrieve positive self-referent words, that is, a relative ease of memory for positive self-referent material (Fletcher et al., 1996; Gould et al., 2003). Such an effect could be mechanistically important for the efficacy of ECT on depressive symptoms including low self-esteem and excessive self-blame.
The absence of effects of ECT on hippocampal activity during retrieval of positive or negative words is consistent with no ECT-related change in limbic activity to emotional faces (Miskowiak et al., 2017). This contrasts with the robust evidence for effects of antidepressant drugs on limbic response to emotional information (Harmer et al., 2006; Norbury et al., 2007; Godlewska et al., 2016). Given this, it is conceivable that ECT primarily affects higher cortical processing of emotional information; in this case, increasing the efficiency of prefrontal retrieval-specific processes for positive information. Indeed, such differential actions of ECT and antidepressant drugs within a key emotion-processing circuitry may contribute to the well-established synergy between these treatments for depression.
Notably, the lack of side-effects of a single ECT session on emotional and nonemotional verbal memory contrasts with patients’ well-documented, burdensome memory impairments after a complete series of ECT that lasts for weeks to months (Semkovska and McLoughlin, 2010; Nordanskog et al., 2014; Bodnar et al., 2016; Mohn and Rund, 2016). This suggests that the side-effects of ECT on verbal memory and hippocampal circuitry result from cumulative rather than acute actions of ECT on neurobiological processes involved in cognition, such as seizure-related excitotoxicity of N-methyl-D-aspartate receptor activation, alterations in cortisol and neurogenesis (Nobler and Sackeim, 2008), and upregulation of oxidative stress (Jorgensen et al., 2013).
The randomized, sham-controlled, parallel-group design was a major strength of the study, which is the first of its kind. The assessment of the effects of a single ECT vs sham session also enabled insight into the early neural activity change that precedes, and may be mechanistically important for, the antidepressant effects of ECT. At the same time, this design was a limitation, since a single ECT session may be insufficient to modulate behavioral assays of negative self-referential memory bias in this heterogeneous patient cohort. However, it would have been unethical to conduct a longer-term randomized controlled study of repeated ECT sessions, since patients were severely ill and 50% received sham. A longitudinal study of the same patients scanned before and after ECT/sham could have supported more robust inferences about the effects of ECT on self-referent memory bias. Nevertheless, the cross-sectional study design was chosen, because repeated testing would have hampered assessment of incidental memory for self-referent words at a second scanning session and is consistent with the approach in our previous studies (Miskowiak et al., 2007). The emotional word recognition task failed to activate the hippocampi. A possible explanation is that patients with mood disorders show deficient recruitment of the hippocampus during memory retrieval (Fairhall et al., 2010; Kelley et al., 2013; Dietsche et al., 2014). Nevertheless, an almost identical self-referent word recognition task also failed to activate the hippocampi in our healthy volunteer reboxetine study (Miskowiak et al., 2007), suggesting that this test might not sufficiently probe hippocampal function. Finally, it is worth noting that approximately 200 patients with nonpsychotic unipolar depression between 18 and 60 years of age (i.e., potentially eligible patients) received ECT at Psychiatric Centre Copenhagen during the 6-year study period (Hundrup et al., 2016). The slow recruitment was mainly a result of lack of manpower (so many patients were randomly missed). Another contributor was the sham-controlled study design since patients scheduled for ECT typically required immediate treatment. This raises the possibility that less acutely unwell patients were selected, although this was not the main mechanism of the slow recruitment.
In conclusion, this randomized, sham-controlled, double-blind fMRI study showed for the first time that a single ECT session reduces the retrieval-specific frontopolar response for positive words in the absence of changes in behavioral performance or mood symptoms. This effect is compatible with early facilitation of memory for positive self-referent information. The finding provides preliminary evidence for modulation of negative self-referent bias in depression as a common mechanism of distinct biological treatments with efficacy on depression.
Acknowledgments
This work was supported by the Lundbeck Foundation (R32-A2904). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Statement of Interest
K.W.M. reports having received consultancy fees from Lundbeck and Allergan in the past 3 years. The Lundbeck Foundation and Weimann Foundation are acknowledged for providing half of K.W.M.’s salary between 2012 and 2018 for her to do full-time research during this time. O.B.P. was a member of the board of directors of the Elsass Foundation until 2016. C.J.H. has received consultancy fees from P1vital ltd, Lundbeck, Servier, and Eli-Lilly and is a company director of Oxford Psychologists ltd. C.J.H. has also received grant income from GSK, UCB, Janssen Inc, Lundbeck, Servier, and Astra Zeneca. Within the past 3 years, M.B.J. has received speaker fees for Lundbeck and consultant fees for Shire. All other authors report no biomedical financial interests or potential conflicts of interest. H.R.S. discloses honoraria as journal editor from Elsevier Publishers and book editor from Springer Publishing as well as honoraria as speaker from Genzyme and MerckSerono, and grant support from Biogen-idec within the past 3 years. L.V.K. reports having been a consultant for Lundbeck, AstraZeneca, and Sunovion within the last 3 years.
References
- Altar CA, Laeng P, Jurata LW, Brockman JA, Lemire A, Bullard J, Bukhman YV, Young TA, Charles V, Palfreyman MG(2004)Electroconvulsive seizures regulate gene expression of distinct neurotrophic signalling pathways. J Neurosci 24:2667–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson NH.(1968)Likeableness of ratings of 555 personality-trait words. J Pers Soc Psychol 9:272–279. [DOI] [PubMed] [Google Scholar]
- Beck AT, Alford BA(2014)Depression causes and treatment. Philadelphia: University of Pennsylvania Press. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J(1961)An inventory for measuring depression. Arch Gen Psychiatry 4:561–571. [DOI] [PubMed] [Google Scholar]
- Bodnar A, Krzywotulski M, Lewandowska A, Chlopocka-Wozniak M, Bartkowska-Sniatkowska A, Michalak M, Rybakowski JK(2016)Electroconvulsive therapy and cognitive functions in treatment-resistant depression. World J Biol Psychiatry 17:159–164. [DOI] [PubMed] [Google Scholar]
- Bolwig TG, Madsen TM(2007)Electroconvulsive therapy in melancholia: the role of hippocampal neurogenesis. Acta Psychiatr Scand 115:130–135. [DOI] [PubMed] [Google Scholar]
- Bouhuys A, Geerts E, Gordijn M(1999)Depressed patients’ perceptions of facial emotions in depressed and remitted states are associated with relapse: a longitudinal study. J Nerv Ment Dis 187:595–602. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ(1996)Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci Off J Soc Neurosci 16:4207–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley B, Mathews A(1983)Negative self-schemata in clinical depression. Br J Clin Psychol Br Psychol Soc 22:173–181. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Lewis PA, Orth M, Josephs O, Deichmann R, Trimble MR, Dolan RJ(2007)Vagus nerve stimulation for treatment-resistant depression: behavioral and neural effects on encoding negative material. Psychosom Med 69:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Simplicio M, Norbury R, Harmer CJ(2012)Short-term antidepressant administration reduces negative self-referential processing in the medial prefrontal cortex in subjects at risk for depression. Mol Psychiatry 17:503–510. [DOI] [PubMed] [Google Scholar]
- Dierckx B, Heijnen WT, van den Broek WW, Birkenhäger TK(2012)Efficacy of electroconvulsive therapy in bipolar versus unipolar major depression: a meta-analysis. Bipolar Disord 14:146–150. [DOI] [PubMed] [Google Scholar]
- Dietsche B, Backes H, Stratmann M, Konrad C, Kircher T, Krug A(2014)Altered neural function during episodic memory encoding and retrieval in major depression: neural function during episodic memory in MDD. Hum Brain Mapp 35:4293–4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson KS, Shaw BF(1987)Specificity and stability of self-referent encoding in clinical depression. J Abnorm Psychol 96:34–40. [DOI] [PubMed] [Google Scholar]
- Dozois DJ, Dobson KS(2001)Information processing and cognitive organization in unipolar depression: specificity and comorbidity issues. J Abnorm Psychol 110:236–246. [DOI] [PubMed] [Google Scholar]
- Fairhall SL, Sharma S, Magnusson J, Murphy B(2010)Memory related dysregulation of hippocampal function in major depressive disorder. Biol Psychol 85:499–503. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Frith CD, Frackowiak RS, Dolan RJ(1996)Brain activity during memory retrieval. The influence of imagery and semantic cueing. Brain J Neurol 119:1587–1596. [DOI] [PubMed] [Google Scholar]
- Godlewska BR, Browning M, Norbury R, Cowen PJ, Harmer CJ(2016)Early changes in emotional processing as a marker of clinical response to SSRI treatment in depression. Transl Psychiatry 6:e957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Castillo J, Roopchansingh V, Bandettini PA, Bodurka J(2011)Physiological noise effects on the flip angle selection in BOLD fMRI. Neuroimage 54:2764–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould R, Brown R, Owen A, ffytche D, Howard R(2003)FMRI BOLD response to increasing task difficulty during successful paired associates learning. Neuroimage 20:1006–1019. [DOI] [PubMed] [Google Scholar]
- Hamilton M.(1960)A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Cowen PJ(2013)‘It’s the way that you look at it’- a cognitive neuropsychological account of SSRI action in depression. 368(1615):20120407. doi: 10.1098/rstb.2012.0407. [DOI] [PMC free article] [PubMed]
- Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM(2006)Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry 59:816–820. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, O’Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, Goodwin GM, Cowen PJ(2009)Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry 166:1178–1184. [DOI] [PubMed] [Google Scholar]
- Hundrup E, Osler M, Jørgensen MB(2016)Time trends and variations in electroconvulsive treatment in Denmark 2008 to 2014: a nationwide register-based study. J ECT doi: 10.1097/YCT.0000000000000381 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Jorgensen A, Krogh J, Miskowiak K, Bolwig TG, Kessing LV, Fink-Jensen A, Nordentoft M, Henriksen T, Weimann A, Poulsen HE, Jorgensen MB(2013)Systemic oxidatively generated DNA/RNA damage in clinical depression: associations to symptom severity and response to electroconvulsive therapy. J Affect Disord 149:355–362. [DOI] [PubMed] [Google Scholar]
- Jorgensen A, Magnusson P, Hanson LG, Kirkegaard T, Benveniste H, Lee H, Svarer C, Mikkelsen JD, Fink-Jensen A, Knudsen GM, Paulson OB, Bolwig TG, Jorgensen MB(2016)Regional brain volumes, diffusivity, and metabolite changes after electroconvulsive therapy for severe depression. Acta Psychiatr Scand 133:154–164. [DOI] [PubMed] [Google Scholar]
- Kelley R, Garrett A, Cohen J, Gomez R, Lembke A, Keller J, Reiss AL, Schatzberg A(2013)Altered brain function underlying verbal memory encoding and retrieval in psychotic major depression. Psychiatry Res Neuroimaging 211:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingström A(2000)Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry 47:1043–1049. [DOI] [PubMed] [Google Scholar]
- Mannie ZN, Bristow GC, Harmer CJ, Cowen PJ(2007)Impaired emotional categorisation in young people at increased familial risk of depression. Neuropsychologia 45:2975–2980. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C(2005)Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol 1:167–195. [DOI] [PubMed] [Google Scholar]
- Miskowiak K, Papadatou-Pastou M, Cowen PJ, Goodwin GM, Norbury R, Harmer CJ(2007)Single dose antidepressant administration modulates the neural processing of self-referent personality trait words. Neuroimage 37:904–911. [DOI] [PubMed] [Google Scholar]
- Miskowiak KW, Carvalho AF(2014)“Hot” cognition in major depressive disorder: a systematic review. CNS Neurol Disord Drug Targets 13:1787–1803. [DOI] [PubMed] [Google Scholar]
- Miskowiak KW, Kessing LV, Ott CV, Macoveanu J, Harmer CJ, Jørgensen A, Revsbech R, Jensen HM, Paulson OB, Siebner HR, Jørgensen MB(2017)Does a single session of ECT alter the neural response to emotional faces in depression? A randomised sham-controlled fMRI study. J Psychopharmacol 31:1215–1224. [DOI] [PubMed] [Google Scholar]
- Mohn C, Rund BR(2016)Significantly improved neurocognitive function in major depressive disorders 6 weeks after ECT. J Affect Disord 202:10–15. [DOI] [PubMed] [Google Scholar]
- Neshat-Doost HT, Taghavi MR, Moradi AR, Yule W, Dalgleish T(1998)Memory for emotional trait adjectives in clinically depressed youth. J Abnorm Psychol 107:642–650. [DOI] [PubMed] [Google Scholar]
- Nobler MS, Sackeim HA(2008)Neurobiological correlates of the cognitive side effects of electroconvulsive therapy. J ECT 24:40–45. [DOI] [PubMed] [Google Scholar]
- Norbury R, Mackay CE, Cowen PJ, Goodwin GM, Harmer CJ(2007)Short-term antidepressant treatment and facial processing: functional magnetic resonance imaging study. Br J Psychiatry 190:531–532. [DOI] [PubMed] [Google Scholar]
- Nordanskog P, Larsson MR, Larsson E- M, Johanson A(2014)Hippocampal volume in relation to clinical and cognitive outcome after electroconvulsive therapy in depression. Acta Psychiatr Scand 129:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel W, Goldin PR, Eyler LT, Brown GG, Gotlib IH, McQuaid JR(2007)Amygdala reactivity and mood-congruent memory in individuals at risk for depressive relapse. Biol Psychiatry 61:231–239. [DOI] [PubMed] [Google Scholar]
- Romero N, Sanchez A, Vazquez C(2014)Memory biases in remitted depression: the role of negative cognitions at explicit and automatic processing levels. J Behav Ther Exp Psychiatry 45:128–135. [DOI] [PubMed] [Google Scholar]
- Schmidt M.(1996)Rey auditory verbal learning test: a handbook. Western Psychological Services Los Angeles. Available at: http://v-psyche.com/doc/Clinical%20Test/Rey%20Auditory%20Verbal%20Learning%20Test.docx Retrieved 29 May, 2016.
- Semkovska M, McLoughlin DM(2010)Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry 68:568–577. [DOI] [PubMed] [Google Scholar]
- Spielberger C.(1983)Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologist Press. [Google Scholar]
- The UK ECT Review Group (2003)Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. The Lancet 361:799–808. [DOI] [PubMed] [Google Scholar]
- van Tol M-J, Demenescu LR, van der Wee NJA, Kortekaas R, Marjan MAN, Boer JAD, Renken RJ, van Buchem MA, Zitman FG, Aleman A, Veltman DJ(2012)Functional magnetic resonance imaging correlates of emotional word encoding and recognition in depression and anxiety disorders. Biol Psychiatry 71:593–602. [DOI] [PubMed] [Google Scholar]
- Watkins PC, Mathews A, Williamson DA, Fuller RD(1992)Mood-congruent memory in depression: emotional priming or elaboration?J Abnorm Psychol 101:581–586. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE(2014)Permutation inference for the general linear model. Neuroimage 92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfensberger SPA, Veltman DJ, Hoogendijk WJG, De Ruiter MB, Boomsma DI, de Geus EJC(2008)The neural correlates of verbal encoding and retrieval in monozygotic twins at low or high risk for depression and anxiety. Biol Psychol 79: 80–90. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM(2001)Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 14:1370–1386. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Okamoto Y, Onoda K, Matsunaga M, Ueda K, Suzuki S, Shigetoyamawaki(2010)Rostral anterior cingulate cortex activity mediates the relationship between the depressive symptoms and the medial prefrontal cortex activity. J Affect Disord 122:76–85. [DOI] [PubMed] [Google Scholar]