Abstract
Background and Aims
The leaf axis of members of the order Cycadales (‘cycads’) has long been recognized by its configuration of independent vascular bundles that, in transverse section, resemble the Greek letter omega (hence the ‘omega pattern’). This provides a useful diagnostic character for the order, especially when applied to paleobotany. The function of this pattern has never been elucidated. Here we provide a three-dimensional analysis and explain the pattern in terms of the hydraulic architecture of the pinnately compound cycad leaf.
Methods
The genus Cycas was used as a simple model, because each leaflet is supplied by a single vascular bundle. Sequential sectioning was conducted throughout the leaf axis and photographed with a digital camera. Photographs were registered and converted to a cinematic format, which provided an objective method of analysis.
Key Results
The omega pattern in the petiole can be sub-divided into three vascular components, an abaxial ‘circle’, a central ‘column’ and two adaxial ‘wings’, the last being the only direct source of vascular supply to the leaflets. Each leaflet is supplied by a vascular bundle that has divided or migrated directly from the closest wing bundle. There is neither multiplication nor anastomoses of vascular bundles in the other two components. Thus, as one proceeds from base to apex along the leaf axis, the number of vascular bundles in circle and column components is reduced distally by their uniform migration throughout all components. Consequently, the distal leaflets are irrigated by the more abaxial bundles, guaranteeing uniform water supply along the length of the axis.
Conclusions
The omega pattern exemplifies one of the many solutions plants have achieved in supplying distal appendages of an axis with a uniform water supply. Our method presents a model that can be applied to other genera of cycads with more complex vascular organization.
Keywords: Cycas revoluta, cycad, omega pattern, anatomy, xylem network, xylem hydraulic architecture
INTRODUCTION
The organization of the vascular system in the leaf axis has long been known as a diagnostic feature of the order Cycadales (Ash, 1985). As seen in transverse section, the arrangement of vascular bundles in the leaf axis is in that of an omega (although in the natural orientation of the leaf the omega is actually inverted; Fig. 1A). The ‘omega pattern’ is known to have a wider distribution in other groups (e.g. Wang et al., 2017), so its function may have wide significance. The cycad leaf axis was studied in some detail by earlier anatomists (e.g. Vetters, 1884; Nestler, 1895), but most extensively by Matte (1904) who described its variation. These early authors were largely concerned with the configuration of the vascular supply to leaflets, which is easily observed externally and, therefore, provides a diagnostic feature (Brashier, 1968; Stevenson, 1990). Matte employed the term ‘méristele’ to refer to the vascular pattern within the axis of a compound leaf. Matte’s work is exemplary in its broad scope and attention to detail, whereby he was able to distinguish examples of increasing complexity, from ‘méristele simple’ to ‘méristele complexe’, among nine of the ten extant genera. In the most divergently different taxa, the omega pattern may not exist at all. His results have subsequently been used in cladistic analysis (Stevenson, 1990). However, in the collective use of the term ‘omega pattern’, although of significance in systematics and phylogeny, authors have not discussed any functional reason for the pattern. In particular, researchers have yet to consider its possible value in sustaining hydraulic continuity, which is a general problem for the water relations in all plant axes that must maintain a continuous water supply against gravity and a increasing resistance from base to apex (Zimmermann, 1983; Tyree and Ewers, 1996; Tyree and Zimmermann, 2002).
Fig. 1.
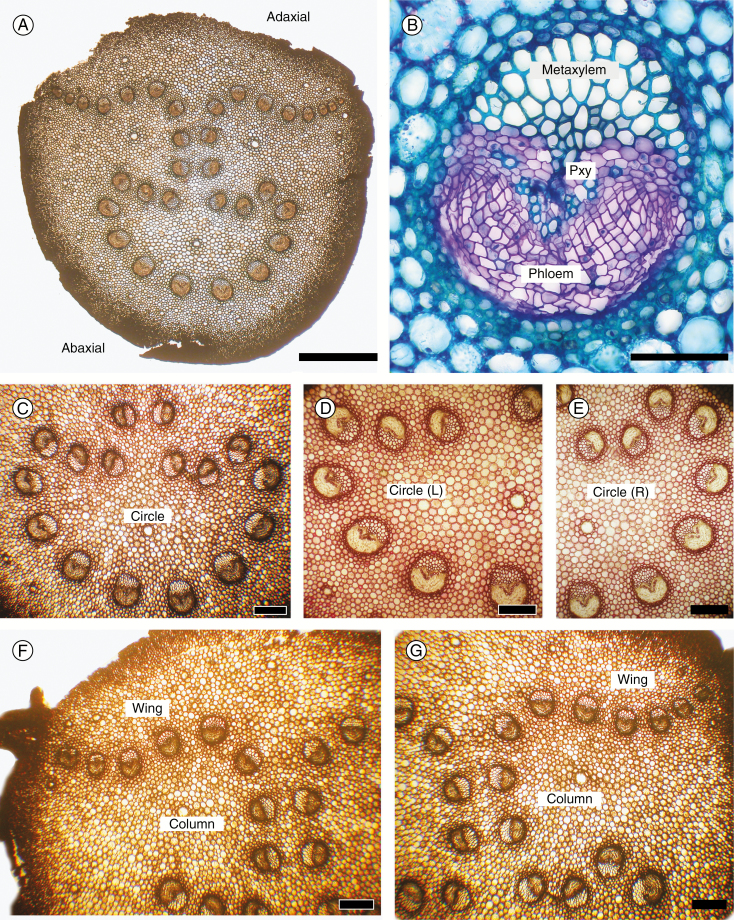
Cycas revoluta leaf axis anatomy. All transverse sections. (A) Petiole base displaying the inverted omega pattern. (B) Single vascular bundle, toluidine blue stain. (C–G) Details of components of the omega pattern; unstained in (C), (F) and (G); stained in phloroglucinol and HCl in (D) and (E) to show the orientation of the metaxylem. (C) Circle bundles. (D) Left circle bundles. (E) Right circle bundles. (F) Detail of column and left wing bundles. (G) Detail of column and right wing bundles. (A) Scale bar = 2 mm; (B) scale bar = 100 µm; (C–G) scale bars = 300 µm.
Here, we analyse the omega pattern in relation to the three-dimensional architecture along the length of the leaf axis. We provide a detailed structural analysis of one species of Cycas, which represents the omega pattern in its simplest form, in order to establish a model that can be used more generally in comparative study among all extant genera. It is appropriate to use Cycas as a model because of its demonstrated status as the outgroup for all extant cycads (Salais-Leiva et al., 2013) and its extensively studied leaflet anatomy (Griffith et al., 2014).
MATERIALS AND METHODS
We used greenhouse and horticultural material of Cycas revoluta as this is the most commonly cultivated species of the genus. Seedling, juvenile and adult leaves were studied and shown to differ only quantitatively, as in the total number of vascular bundles, which is dependent on leaf length.
Sectioning
Sections were cut on a sliding microtome (GSL1, Birmensdorf, Switzerland) at a thickness of 10 and 90 µm, mounted semi-permanently in dilute glycerol and each section was photographed with a digital camera (Canon 6D, Canon, USA) through a ×1 or ×10 objective on a Nikon Eclipse E600 compound microscope (Nikon, USA). Serial sections along the axis and positioned at the node of each leaflet were analysed to track the division and movement of each vascular bundle. A total of five leaves of C. revoluta were analysed. Such histological details as were required involved stains for lignin (phloroglucinol and concentrated HCl) and suberin (Sudan IV) or the use of toluidine blue (0.01 %) as a metachromatic stain on sections first bleached in sodium hypochlorite.
Analysis
Images were stacked and registered in sequential order using Adobe Photoshop CC (ver. 14.2.1 × 64; Adobe Systems, San Jose, CA, USA). Layers were converted to a cinematic format set at 0.5 or 1.0 s per frame (Huggett and Tomlinson, 2010) (Supplementary Data Videos S1 and S2). When converted into animation, a totally objective and repeatable record is available. Transverse sections were illustrated with colour coding of the various components to display the gradual transformation of the vascular architecture proceeding from the base to apex of the leaf axis (Fig. 2). The mechanical tissue is often disrupted by the sectioning of the unembedded material, often resulting in incomplete peripheral regions, but the consistency of the vascular system throughout the full length of the axis is all that is required.
Fig. 2.
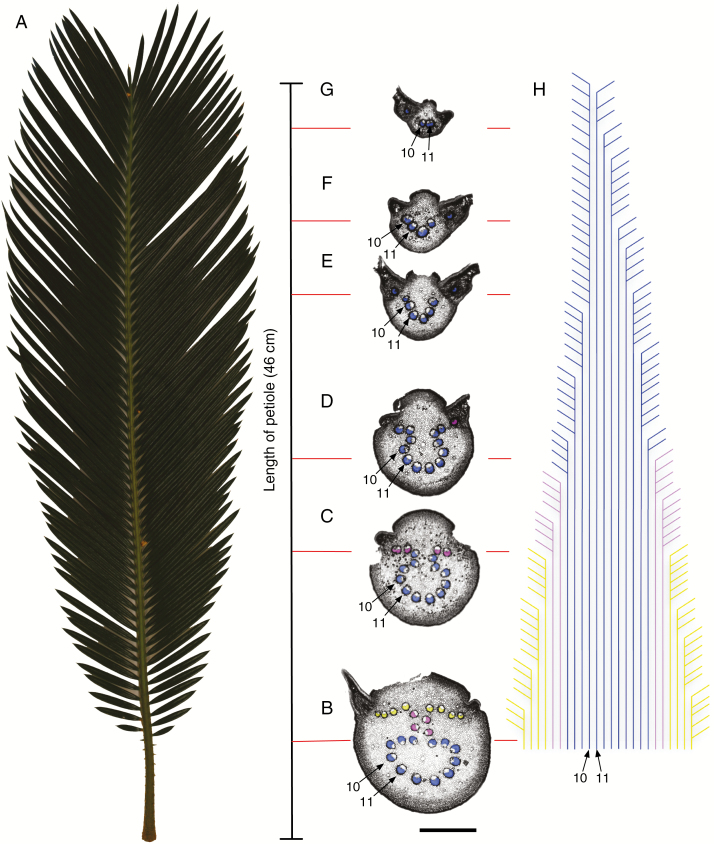
The vascular architecture of Cycas revoluta. (A) C. revoluta leaf; (B–G) transverse sections selected from 125 serial sections along the rachis to illustrate the gradual transformation of the bundles in the distal direction; blue, circle bundles; purple, column bundles; yellow, wing bundles; bundles were numbered from left to right, with numbers 10 and 11 indicated for orientation through the series; scale bar = 2 mm. (H) Illustration of the course of vascular bundles in the distal direction indicating the division and exiting of vascular bundles occupying the most distal wing position in the rachis; colours and numbers correspond to vascular bundles in (B–G).
RESULTS
Histology
A transverse section of the petiole (Fig. 1A) shows a peripheral series of lignified fibres that constitute the chief mechanical tissue of the leaf axis. The parenchymatous ground tissue is distinguished in this species by its conspicuously pitted walls, although they remain unlignified. Mucilage canals form a peripheral series, with a few more scattered in the centre. The vascular bundles are of uniform composition and entirely primary in construction (Fig. 1B), each with a mesarch xylem arrangement in which the bulk of the tracheids are centrifugal in origin. These constitute the chief transport system of the bundle because they are abundant and wide. The orientation of this metaxylem varies throughout the length of most vascular bundles in an ordered way, as detailed below. A distinctive morphological feature of the genus Cycas is the lateral series of spiny petiolar outgrowths that represent highly reduced leaflets. Each is supplied by a short, vestigial vascular bundle, which corresponds to the single vascular bundle of each leaflet in the rachis. In all other genera of cycads, each leaflet is supplied by several vascular bundles (Matte, 1904), and petiolar spines are rarely developed.
Vascular system
The vascular arrangement in the leaf axis (mériphyte of Matte, 1904) is seen in a transverse section of the petiole as a convoluted series of independent vascular bundles (Fig. 1A; the omega pattern), which we followed distally through the length of the leaf axis. In the petiole portion of the leaf axis, it is convenient to divide the vascular system into three categories (Fig. 1C–G). First is the abaxial series that forms an incomplete circle (hence ‘circle bundles’) in which the abaxial members have a normal orientation as determined by the location of the metaxylem (Fig. 1C; circle bundles). However, there is a progressive change in metaxylem location so that in the adaxial direction bundles are initially seen as oblique (Fig. 1D, E) and then with an inverted orientation (upper bundles Fig. 1C, D). Secondly, in the middle of the system, is a short columnar series (hence ‘column bundles’) of paired vascular bundles with the metaxylem oriented horizontally, with the opposed bundles facing each other (Fig. 1F, G). In the adaxial part of the system are two lateral series of horizontal bundles, left and right (Fig. 1F, G; ‘wing bundles’). These bundles are progressively substituted by the lower vascular bundles proceeding from the base to the apex of the leaf axis as illustrated in Fig. 2. The wing bundles are progressively narrower towards the outer margin of the wing and their numbers vary at different levels because they are the only bundles that divide. Each leaflet is supplied by a vascular bundle that has divided or migrated directly from the most marginal wing bundle.
Omega pattern – petiole
The pattern within the petiole, i.e. the portion of the leaf axis below the insertion of the leaflets, shows no change throughout its length, because there is neither anastomosing nor branching within the main system (Fig. 2A). Vascular bundles have been labelled and colour-coded to facilitate recognition at each successive level from the base of the leaf axis to the apex (Fig. 2B–H; circle bundles, blue; column bundles, purple; wing bundles, yellow). Vascular bundles number 10 and 11 are selected as a point of reference as they are continually present in all sections along the leaf axis (Fig. 2B–I). Continued recognition of bundles at all levels is guaranteed by sequential sectioning, and the course of vascular bundles can be mapped out in relation to each leaflet along the rachis (Fig. 2H).
Omega pattern – rachis
The division and exiting of wing bundles from the rachis, i.e. the portion of the leaf axis with the insertion of the leaflets, in addition to the movement of column and circle bundles into the wing position as one proceeds along the rachis is illustrated in Fig. 2B–H. The distal portion of the leaf axis, on which the leaflets are inserted, shows progressive reduction in the number of vascular bundles and the gradual loss of the omega pattern (Fig. 2B–H). Because our description is based on cinematic analysis, verbs of motion are appropriate for the changes in the axis that are only positional, as we refer to the continual supply of traces to leaflets, one for each leaflet, and their incomplete replacement by bundle branching. The underlying mechanism is the progressive displacement of circle and column bundles in the distal direction. This displacement moves circle bundles into the columnar series (Fig. 2C–E) and columnar bundles into the wing series (Fig. 2C), until only circle bundles remain (Fig. 2E–G). Changes in bundle orientation, determined by the position of the vascular tissues, are most obvious in the metaxylem (Fig. 1C–G). At first this tissue in the most abaxial circle bundles is adaxial, a normal orientation, but then becomes inverted, then horizontal, and finally rotated back to abaxial in the wing. Leaflets are progressively supplied one at a time by the outermost bundles of the wing series. These wing bundles are in part replaced by bundle division within the series, but largely by the replacement of bundles at the centre, originally derived from the column series and ultimately the circle series (Fig. 2C–G).
Every bundle can be followed by its colour code, and with branching and exiting into leaflets illustrated in Fig. 2H. The exiting of a vascular bundle from the rachis into a leaflet is observed in Fig. 2D, where a vascular bundle originating in the columnar section (coloured in purple) can be seen moving from the wing position into the leaf trace. The overall loss of bundles gradually modifies the omega pattern, leading to a U-shaped series of bundles (as a horse-shoe pattern, (Fig. 2C), then a U (Fig. 2E) and finally a V (Fig. 2F), with leaflet supply always from its upper margin. The terminus of the system is thus supplied eventually by bundles that were originally members of the circle system as with bundle numbers 10 and 11 (Fig. 2B–G).
Bundle numbers
The configuration of vascular bundles along the leaf axis can be quantified in relation to the total number of bundles at any one level and their gradual disappearance, because each leaflet receives only one axial vascular bundle. Consequently, a count of the total leaflets along the axis subtracted by the number of vascular bundles at any one level gives the number of bundles that must be increased beyond that level. For example, the number of vascular bundles in Fig. 2, which represents the base of the leaf axis, is 24, but beyond this 125 leaflets (left and right) are supplied. Therefore, 101 new bundles must be added to supply all the leaflets. This additional number is supplied, not by any increase in number in the circle and column systems, but entirely by proliferation in the vascular bundles occupying the wing system, as charted by Matte (1904). There appears to be no pattern to the number of times a vascular bundle occupying the wing system will divide before migrating into a leaflet. Serial sectioning along the entire rachis reveals that wing bundles may divide as little as once or as many as 18 times (Fig. 2H).
Hydraulics
One may reasonably translate the pathway for water movement along the leaf axis in terms of the distribution of vascular bundles and the metaxylem within them. Conclusions can be drawn from the diagrammatic images of Fig. 2. In the petiole, the omega is pronounced and maintained throughout its length without anastomosing or branching of individual bundles. Each bundle therefore serves as an independent unit of supply. In the rachis, progressive reduction in vascular bundle number occurs along the axis in an ordered sequence from the three components we have identified, from circle, to column to wing. In this progression, the omega pattern is lost and eventually only ‘circle’ bundles remain and then disappear. Leaflets are all supplied directly from a single vascular bundle, each of which diverges as a branch from the outermost and most adaxial bundles, initially the outermost wing bundles, and subsequently from any bundle that comes to occupy this position, having passed through proximal sources. Most significantly, the distal leaflets are supplied by the most abaxial of the circle bundles, which are the longest uninterrupted bundles of the whole méristele. In this way, a water supply to the seemingly most disadvantaged distal leaflets is guaranteed. This is seen in the diagram of Fig. 2 where bundles numbers 10 and 11, abaxial bundles, have the longest uninterrupted pathway, traceable throughout the whole axis. Vascular bundle 10 finally becomes the last bundle to supply the most distal leaflets. From this, we can conclude that the omega pattern exists as a structural feature that allows water movement through the leaf axis in an ordered and efficient manner.
DISCUSSION
The analysis we present should be placed in the context of hydraulic architecture of plants, a concept generated by Martin H. Zimmermann (Zimmermann, 1983; Tyree and Zimmermann, 2002), in which the trade-off between safety and efficiency in xylem transport of water must be assessed against the structural architecture of plants. Here water must be raised through an interconnected system, against gravity and the resistance within the conduits themselves. In plants, this means that distal parts of any axial system are at a disadvantage, and water supply to lateral appendages cannot be detrimental to continued axial supply itself. A good example was provided by the vascular system of arborescent monocotyledons with exclusively primary conducting tissues, such as palms. It was shown that the vascular continuity in the main axis (trunk) of the palm Rhapis excelsa is maintained by an interconnected system of wide metaxylem vessels, whereas the supply to lateral appendages (notably leaves) was provided by narrow protoxylem tracheids (Zimmermann and Sperry, 1983). This separation had an explanation in the method of development of the system (Zimmermann and Tomlinson, 1967). In this way, structure, development and function were unified in a common biological framework.
Our analysis of the omega pattern in cycad leaves is still incomplete in developmental detail, but does show that the pattern is consistent with a principle of protection of an axial system against the requirements of an appendicular supply. A large number of axial vascular bundles with well-developed metaxylem tracheids supply laterally disposed leaflets via narrow leaflet traces that have a uniform disposition, essentially at the extremity of the total vascular framework. The axial system maintains continuity in such a way that as the number of vascular bundles decreases axially in a necessary determinate fashion as the rachis tapers a distal supply is maintained. The distal-most leaflets are supplied by the longest axial bundles, whose sectored pathway is uninterrupted either by division or by anastomosis. We offer this as an explanation of the configuration that results in an omega-shaped vascular system. Furthermore, this sectored vascular architecture of the omega pattern in cycad leaves could result in more efficient water transport. Research on ferns with similar compound leaf arrangements has shown a strong correlation between safety and efficiency in terms of hydraulic transport (Brodersen et al., 2012). In comparing the integrated xylem networks of Pteridium aquilinium with that of the sectored networks of Woodwardia fimbriata, Brodersen et al. (2012) found that the more sectored vascular architecture in W. fimbriata was associated with higher cavitation resistance. Similar trends in the vascular sectorality and hydraulic efficiency may also occur in plants within Cycas.
The earlier work on this topic had a different approach from ours, as it considered only the distal portion of the mériphyte in relation to the insertion of the leaflets and consider its variation in different genera. To do this, these previous authors described the sequences observed only in a basal direction, i.e. from the leaflet base into the rachis. This obviously is in the direction opposite to that in which water would flow. Furthermore, in the three-dimensional construction of the vascular pathway, early authors followed the method instigated by Vetters (1884) by showing, in a single plane, only the immediate connection of a leaflet to the axis. Matte (1904) reproduced a diagram from the paper by Nestler (1895) who first copied this method. Followed outwards, this would imply both branching and fusion of leaflet traces, which occurs only, at most, in the wing bundles in the system we have devised. These authors indicate little three-dimensional analysis of the omega pattern as a whole, even though some illustrations of sections showed the pattern in the more basal parts of the axis. Our analysis of the entire system shows that in Cycas the axial bundles remain independent in the circle and column series, the central component of the hydraulic pathway, and only in a progressive distal direction do they become components of the system that supplies the leaflets. Nevertheless, the work of these early authors is important as it drew attention to the diversity to be observed in what is termed the ‘mériphyte complexe’ and the apparent absence of the omega pattern in some genera. Our description is therefore of only the simplest model but can be followed in a further comparative study of the ‘meriphyte’. This is likely to be a considerable task, involving all genera and more species from one genus.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consists of the following. Video S1: animation of sequential sections over a 2 cm length of the rachis to illustrate division and exiting of axial wing bundles into leaflets. Video S2: animation of sequential sections over the entire rachis (46 cm) to illustrate changes in the ‘omega’ vascular architecture.
ACKNOWLEDGEMENTS
We thank Mary Hughes, Bates College and Tonina Burnham for cultivation of specimens. This research was supported by funding from the Bates College Biology Department, 44 Campus Avenue, Lewiston, ME 04240, USA.
LITERATURE CITED
- Ash SR. 1985. A short thick cycad stem from the Upper Triassic of Petrified Forest National Park, Arizona, and Vicinity. Museum of Northern Arizona Bulletin 54: 17–32. [Google Scholar]
- Brashier CK. 1968. Vascularization of cycad leaflets. Phytomorphology 18: 35–42. [Google Scholar]
- Brodersen CR, Roark LC, Pitterman J. 2012. The physiological implications of primary xylem organization in two ferns. Plant, Cell and Environment 35: 1898–1911. [DOI] [PubMed] [Google Scholar]
- Griffith MP, Magellan TM, Tomlinson PB. 2014. Variation in leaflet structure in Cycas (Cycadales – Cycadaceae): does anatomy follow phylogeny and geography?International Journal of Plant Science 175: 241–255. [Google Scholar]
- Huggett BA, Tomlinson PB. 2010. Aspects of vessel dimensions in the aerial roots of epiphytic Araceae. International Journal of Plant Science 171: 362–369. [Google Scholar]
- Matte H. 1904. Recherches sur l’appareil libero-ligneux des Cycadacées. Thesis, Caen: (Reprinted in Memoires de la Societé Linnaean de Normandie ser. 6). [Google Scholar]
- Nestler A. 1895. Ein Beitrag zur Anatomie der Cycadeenfiedern. Jahrbuch der Wissenschaftlichen Botanik 27: 341–368. [Google Scholar]
- Salas-Leiva D, Meerow AW, Calonje M et al. . 2013. Phylogeny of the cycads based on multiple single copy nuclear genes: congruence of concatenation and species inference methods. Annals of Botany 112: 1263–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SJ, Bateman RM, Spencer AR, Wang J, Shao L, Hilton J. 2017. Anatomically preserved ‘strobili’ and leaves from the Permian of China (Dorsalistachyaceae fam. nov.) broaden knowledge of Noeggerthiales and constrain their possible taxonomic affinities. American Journal of Botany 104: 127–149. [DOI] [PubMed] [Google Scholar]
- Stevenson DW. 1990. Morphology and systematics of the Cycadales. Memoirs of the New York Botanical Garden 57: 8–55. [Google Scholar]
- Tyree M, Ewers FW. 1996. Hydraulic architecture of woody tropical plants. In: Mulkey SS, Chazdon RL, Smith AP, eds. Tropical forest ecophysiology. New York: Chapman & Hall, 217–243. [Google Scholar]
- Tyree M, Zimmermann MH. 2002. Xylem structure and the ascent of sap, 2nd edn. Heidelberg: Springer Verlag. [Google Scholar]
- Vetters KL. 1884. Die Blattstiele der Cycadeen. Thesis, Leipzig. [Google Scholar]
- Zimmermann MH. 1983. Xylem structure and the ascent of sap.Berlin: Springer-Verlag. [DOI] [PubMed] [Google Scholar]
- Zimmermann MH, Sperry J. 1983. Anatomy of the palm Rhapis excelsa. IX. Xylem structure of the leaf insertion. Journal of the Arnold Arboretum 64: 599–609. [Google Scholar]
- Zimmermann MH, Tomlinson PB. 1967. Anatomy of the palm Rhapis excelsa. IV. Vascular development in apex of vegetative aerial axis and rhizome. Journal of the Arnold Arboretum 48: 122–142. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.