Abstract
Background:
Gestational diabetes mellitus (GDM) is defined as a carbohydrate intolerance first diagnosed in pregnancy and may be associated with adverse maternal and perinatal outcome.
Aim:
The aim of the study was to determine the maternal and perinatal outcome in GDM during pregnancy.
Materials and Methods:
It is a retrospective analysis of women diagnosed with GDM who got antenatal care and delivered in our hospital in previous 5 years. Another 191 women with normal pregnancy without GDM and other medical conditions were taken as control. The baseline characteristics (age, body mass index, religion, and socioeconomic status) were noted in all cases. Diagnosis of GDM was made using oral glucose tolerance test with 75 g glucose. GDM patients were started on diet following which insulin or oral hypoglycemic agents were given if required. Maternal and perinatal outcome was noted in all women.
Results:
The prevalence of GDM was 5.72% (170/2970). Most patients (79.41%) could be controlled on diet alone. However, 21 (12.35%) needed insulin and 14 (8.23%) needed oral hypoglycemic agents. Middle socioeconomic status was more common in GDM than control and pregnancy-induced hypertension was more common in GDM (13.5%) than in control (6.3%) (P = 0.019). Mode of delivery was not different in two groups. Instrumental deliveries and postpartum hemorrhage were also similar. However, mean birth weight was significantly higher in GDM (2848 ± 539 g) than in control (2707 ± 641 g) (P = 0.004). Incidence of large-for-date babies was also higher (28.2%) in GDM than control (19.4%) (P = 0.005). In neonatal complication, hypoglycemia was significantly higher in GDM (20.6%) than in control (5.2%) (P = 0.001). However, the incidence of hyperbilirubinemia and congenital malformations was not significantly different in two groups.
Conclusion:
The prevalence of GDM was 5.72% in this study. Adequate treatment of GDM on diet, oral hypoglycemic agents, or insulin to achieve euglycemia can achieve near-normal maternal and neonatal outcome.
Keywords: Gestational diabetes mellitus, oral glucose tolerance test, perinatal complication, prevalence
INTRODUCTION
As per the World Health Organization, gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with onset or first recognized during pregnancy.[1] It is a common problem with prevalence varying from 2% to 22% of all pregnancies due to the use of different criteria for diagnosis.[2] GDM constitutes 90%–95% of all cases of diabetes seen in pregnant women.[3] There are controversies about screening, diagnostic tools, and glucose level threshold use as different organizations use different criteria.[2]
Many studies report maternal and fetal complication with GDM but were flawed due to a number of confounding factors such as obesity, older maternal age, and various other comorbidities.[4] Most convincing evidence of adverse pregnancy outcome in gestational diabetes was provided by hyperglycemia and adverse pregnancy outcome (HAPO).[5] After this study, in 75 g oral glucose tolerance test (GTT) fasting ≥92 mg, 1 h ≥180 mg/dl, and 2 h ≥153 mg/dl plasma glucose values (any single value more than the mentioned limit) are taken as GDM.[6] In India, Seshiah et al. performed a community-based study on the prevalence of GDM in South India and came up with Indian guidelines for GDM which are commonly used in Indian condition.[7]
MATERIALS AND METHODS
It was a retrospective cohort study of 170 GDM patients who were managed and delivered in a tertiary care center in New Delhi over a period of 5 years (January 2011–January 2016). Another 191 women with normal profile patients without GDM who delivered during the same time were taken as controls. Baseline characteristic of women including age, body mass index (BMI), socioeconomic status, and religion was recorded.
Diagnosis of GDM was made by GTT using 75 g glucose. Patient was labeled as GDM if any one value is more than criteria (fasting blood sugar [BS] ≥92 mg/dl, 1 h BS ≥180 mg/dl, and 2 h BS ≥153 mg/dl). Initially, patients were started on diabetic diet with some physical exercises. Diet was started by a dietician. If BS levels were not controlled on diabetic diet, then women were either started on oral hypoglycemic agent or insulin in collaboration with endocrinologist.
The women received regular antenatal care. All antenatal investigations were performed. All women were screen for Down's syndrome using Level I ultrasound and dual screen followed by triple screen. Level II ultrasound (anomaly screen) was performed at 18–20 weeks in all patients. Any antenatal complications were noted and treated, particularly urinary tract infection (UTI), candidiasis, preeclampsia, polyhydramnios, etc.
As a protocol, all patients with GDM on insulin were induced at 38 weeks, and those controlled on diet were induced at 40-week period of gestation.
Statistical analysis
All data analyses were carried out using statistical product services solution IBM SPSS version 20.0, IBM Corp. in Armonk, NY. Test of normality assumption of continuous data was done using appropriate statistical test. For normally distributed continuous variables, descriptive statistics such as mean, standard deviation, and the range values were calculated. Comparison of two group means was tested using Student's t-independent test. For nonnormal data, median values and interquartile range were computed. Median values were compared using nonparametric Mann–Whitney U-test. For categorical variables, data were presented as frequency and percent values. Frequency data across categories were compared using Chi-square/Fisher's exact test as appropriate. A two-sided probability of P < 0.05 was considered statistically significant for all statistical tests.
RESULTS
Out of a total of 2970 women who delivered from January 2011 to January 2016, 170 women developed GDM as per criteria using 75 g GTT making prevalence to be 5.7% (98% confidence interval; 4.9–6.5).
Table 1 summarizes the method of diagnosing GDM [Table 1a] and treatment received by the patient [Table 1b]. A total of 135 (79.41%) were controlled on diet, whereas 21 (12.35%) required insulin and 14 (8.23%) were treated with oral hypoglycemic agent (metformin).
Table 1.
(a) Method of diagnosis (b) Modes of treatment for gestational diabetes mellitus
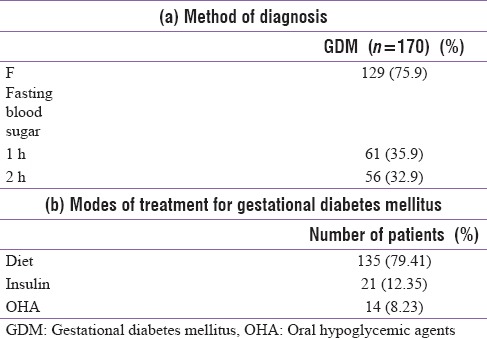
Baseline characteristic of diabetic women and control is shown in Table 2. There was no significant difference in age, BMI, and religion in both groups. However, there was a significant difference in socioeconomic status with a significantly higher number of women in middle socioeconomic class in GDM (63.5%) as compared to control (46.6%) (P = 0.001). Family history of diabetes was observed in a significantly higher number of GDM patients (22.4%) as compared to control group (10%) (P = 0.002).
Table 2.
Baseline characteristics of patients and control
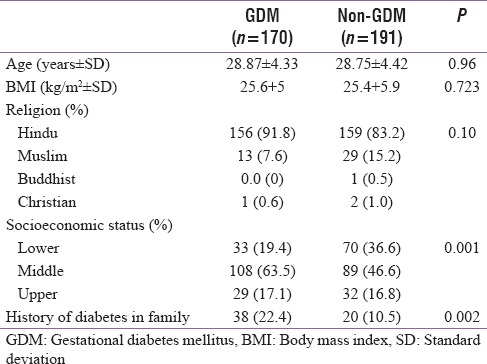
Various antenatal complications of two groups are shown in Table 3. Gestational hypertension and preeclampsia (pregnancy-induced hypertension) were seen in a significantly higher number of cases in GDM patients as compared to controls (13.5% vs. 6.3%) (P = 0.019) whereas polyhydramnios was also seen in higher number in GDM (1.2% vs. 0%, P = 0.22). Prevalence of other antenatal complications such as UTI and candidiasis was similar in two groups.
Table 3.
Antenatal complications in gestational diabetes mellitus and nongestational diabetes mellitus patients

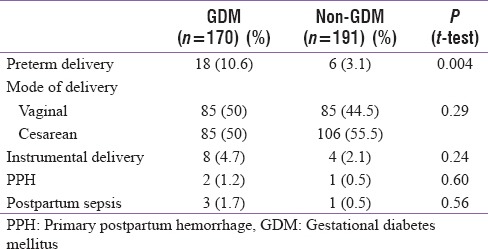
Obstetric outcome in two groups is shown in Table 4. Preterm delivery rate was higher (10.6%) in GDM patients as compared to control group (3.1%) (P = 0.004). There was no significant difference in the mode of delivery between the two groups (P = 0.29). Postpartum hemorrhage (P = 0.60) and postpartum complication (P = 0.56) were also similar in two groups.
Table 4.
Obstetric outcome in 2 groups
Perinatal outcome and neonatal complication in the two groups are shown in Table 5. Mean birth weight was significantly higher in (2848.8 ± 539.4 g) GDM group as compared to control (2707.5 ± 648.4 g) (P = 0.04). There was no significant difference in Apgar score at 1 and 5 min in two groups. There was a significantly higher number of large-for-date babies in GDM group (28.2%) as compared to control group (19.4%) (P = 0.003).
Table 5.
Perinatal outcome and neonatal complications in two group
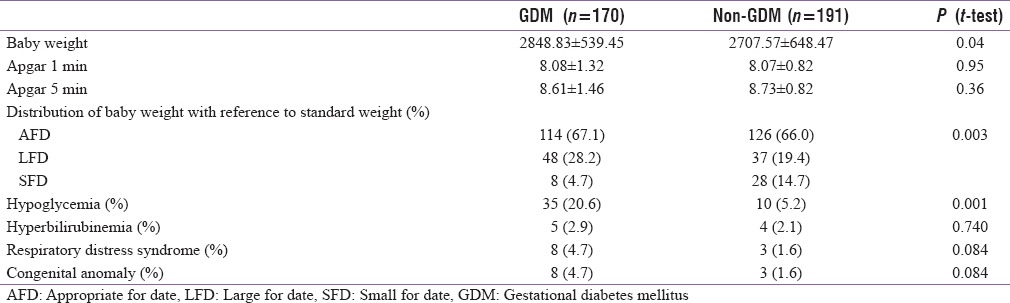
Neonatal complications [Table 5] such as hypoglycemia was seen in significantly higher number of cases in GDM group (20.6%) as compared to control group (5.2%) (P = 0.001). However, incidence of hyperbilirubinemia and congenital malformations was not significantly different in two groups.
DISCUSSION
Gestational diabetes mellitus (GDM) is common problem in pregnancy.[1,2] Overt diabetes mellitus is well known to have adverse antenatal and neonatal outcome. However, controversies exist regarding adverse effects of GDM due to the use of different criteria used by different studies and various confounding factors in these studies.[4] However, the HAPO study confirmed adverse maternal and fetal outcome with rising blood glucose levels in the form of large for date, cesarean delivery rate, and neonatal hypoglycemia as a primary outcome and preeclampsia, preterm delivery, shoulder dystocia, birth injury, hyperbilirubinemia, and intensive neonatal care as secondary outcome. All primary outcome and secondary outcome were affected with maternal hyperglycemia and the prevalence of complication was directly proportional to rising blood glucose levels.[5] Most guidelines have been developed taking results of HAPO study in consideration including Indian guidelines by Seshiah et al.[7,8]
The incidence of GDM in the present study was found to be 5.72% which was lower than that of 13% by Nair et al.[9] from Kolkata, Bengaluru, and Pune and similar to 7.17% by Rajput et al.[10] from Rohtak, Haryana and higher than that of 3.8% by Zargar et al.[11] from Kashmir. However, Seshiah et al.[8] in a study found the prevalence of GDM to be very high being 17.8% in urban, 13.8% in semiurban, and 9.9% in rural area of Tamil Nadu. In the present study, GDM was found to be higher in middle and upper socioeconomic class, but Rajput et al. observed higher prevalence in low socioeconomic class.[10] History of diabetes in family was significantly higher in GDM cases in the present study as compared to controls. Similar results were obtained by Nair et al.[9]
In the present study, antenatal complications such as gestational hypertension and preeclampsia were significantly higher as compared to controls. The results are similar to Nair et al.[9] and HAPO study.[5]
In the present study, there was no significant difference in mode of delivery (cesarean delivery and instrumental delivery) in GDM as compared to controls, an observation also reported by HAPO study[6] and Nair et al.[9]
In perinatal outcome, mean birth weight was significantly higher (2848.83 ± 539.95 g) in GDM cases as compared to controls (2707.57 ± 648.43 g) (P = 0.04). Similarly, large-for-date babies were significantly higher in GDM patients than control (28.2% vs. 19.4%, P = 0.003). There was significantly higher incidence of neonatal hypoglycemia in GDM patients than control (20.6% vs. 5.2%, P = 0.001). However, there was no significant difference in Apgar scoring, congenital malformation, and neonatal hyperbilirubinemia in the two groups. The results were similar to that of Nair et al.[9] and Djomhou et al. from Cameroon,[2] who observed increased incidence of macrosomia in their study. Other authors and a systematic review of WHO and International association of diabetes and pregnancy study group of India diagnostic criteria observed adverse maternal and perinatal outcome, especially macrosomia and neonatal hypoglycemia in GDM patients as compared to controls.[12,13,14]
In a Californian, study by Sacks et al.[15] found prevalence of GDM to be 17.8% (9.3%–25.5%) and adverse perinatal outcome in these patients. In another study from New York, USA, Most et al.[16] observed adverse perinatal outcome in women diagnosed to have GDM in the early pregnancy, and the adverse pregnancy outcome was present despite early identification and management of GDM due to greater severity of disease.[9,16]
In a study conducted in diabetes care center in Chennai, India, using Diabetes in Pregnancy Study Group of India criteria, Balaji et al.[17] observed an incidence of 13.4% of GDM in pregnancy and need of insulin to be in 9.7% which was similar to need of insulin in 12.35% in our study. Nair et al.[9] observed most complication including macrosomia, fetal distress, birth injuries, and dystocia could be reduced significantly by adequate glycemic control in the antenatal period. We also observed very slight increase in parameters including large-for-date babies, birth weight, and neonatal hypoglycemia in GDM patients but most other parameters such as mode of delivery, neonate Apgar, and instrumental deliveries were similar in the two groups due to adequate control of BSs by diet control, insulin, and oral hypoglycemic agents. Similar observation was made by Kwik et al.[18] Similarly, respiratory distress syndrome and hyperbilirubinemia in the present study were similar to control levels due to proper control of GDM by maintaining euglycemia and using maternal steroid for fetal pulmonary maturation in women at risk of premature babies. Mitanchez et al.[19] observed that untreated moderate or severe GDM increased the risk of fetal and neonatal complications. However, the risk of neonatal complication and macrosomia was minimal with adequate treatment. They found a relationship between maternal blood glucose levels and increased birth weight. Treatment of GDM reduces the risk of macrosomia and adverse neonatal outcome.
CONCLUSION
There is a higher prevalence of GDM in India which varies from area to area and socioeconomic status. Adequate treatment of GDM on diet, oral hypoglycemic agents, or insulin to achieve euglycemia can achieve near-normal maternal and neonatal outcome. Although birth weight and neonatal hypoglycemia remain higher in GDM patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.World Health Organization. The Global Burden of Disease 2000. Geneva: World Health Organization; 2006. [Last accessed on 2017 Aug 16]. Available from: https://www.google.co.in/search?q=World+Health+Organization.+The+global+burden+of+disease+2000.+Geneva:+WHO%3B+2006.&ie=utf-8&oe=utf-8&gws_rd=cr&ei=uk-UWdLWGMT3vgT5_aOQCA. [Google Scholar]
- 2.Djomhou M, Sobngwi E, Noubiap JJ, Essouma M, Nana P, Fomulu NJ, et al. Maternal hyperglycemia during labor and related immediate post-partum maternal and perinatal outcomes at the Yaoundé Central Hospital, Cameroon. J Health Popul Nutr. 2016;35:28. doi: 10.1186/s41043-016-0065-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Preventive Services Task Force. Screening for gestational diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148:759–65. doi: 10.7326/0003-4819-148-10-200805200-00008. [DOI] [PubMed] [Google Scholar]
- 4.Weinert LS. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy: Comment to the International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Diabetes Care. 2010;33:e97. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 6.>HAPO Study Cooperative Research Group. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: Associations with neonatal anthropometrics. Diabetes. 2009;58:453–9. doi: 10.2337/db08-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seshiah V, Sahay BK, Das AK, Shah S, Banerjee S, Rao PV, et al. Gestational diabetes mellitus – Indian guidelines. J Indian Med Assoc. 2009;107:799. [PubMed] [Google Scholar]
- 8.Seshiah V, Balaji V, Balaji MS, Paneerselvam A, Arthi T, Thamizharasi M, et al. Prevalence of gestational diabetes mellitus in South India (Tamil Nadu) – A community based study. J Assoc Physicians India. 2008;56:329–33. [PubMed] [Google Scholar]
- 9.Nair VG, Sandhu GS, Biswas M, Bhalla R. Evaluation of the incidence and outcome of gestational diabetes mellitus using the current international consensus guidelines for diagnosing hyperglycaemia in pregnancy. Int J Reprod Contracept Obstet Gynecol. 2016;5:3361–6. [Google Scholar]
- 10.Rajput R, Yadav Y, Nanda S, Rajput M. Prevalence of gestational diabetes mellitus & associated risk factors at a tertiary care hospital in Haryana. Indian J Med Res. 2013;137:728–33. [PMC free article] [PubMed] [Google Scholar]
- 11.Zargar AH, Sheikh MI, Bashir MI, Masoodi SR, Laway BA, Wani AI, et al. Prevalence of gestational diabetes mellitus in Kashmiri women from the Indian subcontinent. Diabetes Res Clin Pract. 2004;66:139–45. doi: 10.1016/j.diabres.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Wahi P, Dogra V, Jandial K, Bhagat R, Gupta R, Gupta S, et al. Prevalence of gestational diabetes mellitus (GDM) and its outcomes in Jammu region. J Assoc Physicians India. 2011;59:227–30. [PubMed] [Google Scholar]
- 13.Nilofer AR, Raju VS, Dakshayini BR, Zaki SA. Screening in high-risk group of gestational diabetes mellitus with its maternal and fetal outcomes. Indian J Endocrinol Metab. 2012;16(Suppl 1):S74–8. doi: 10.4103/2230-8210.94268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wendland EM, Torloni MR, Falavigna M, Trujillo J, Dode MA, Campos MA, et al. Gestational diabetes and pregnancy outcomes – A systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth. 2012;12:23. doi: 10.1186/1471-2393-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sacks DA, Hadden DR, Maresh M, Deerochanawong C, Dyer AR, Metzger BE, et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care. 2012;35:526–8. doi: 10.2337/dc11-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Most OL, Kim JH, Arslan AA, Klauser C. Maternal and neonatal outcomes in early glucose tolerance testing in an obstetric population in New York city. J Perinat Med. 2009;37:114–7. doi: 10.1515/JPM.2009.034. [DOI] [PubMed] [Google Scholar]
- 17.Balaji V, Balaji M, Anjalakshi C, Cynthia A, Arthi T, Seshiah V, et al. Diagnosis of gestational diabetes mellitus in Asian-Indian women. Indian J Endocrinol Metab. 2011;15:187–90. doi: 10.4103/2230-8210.83403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwik M, Seeho SK, Smith C, McElduff A, Morris JM. Outcomes of pregnancies affected by impaired glucose tolerance. Diabetes Res Clin Pract. 2007;77:263–8. doi: 10.1016/j.diabres.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Mitanchez D. Foetal and neonatal complications in gestational diabetes: Perinatal mortality, congenital malformations, macrosomia, shoulder dystocia, birth injuries, neonatal complications. Diabetes Metab. 2010;36:617–27. doi: 10.1016/j.diabet.2010.11.013. [DOI] [PubMed] [Google Scholar]


