Fig. 1.
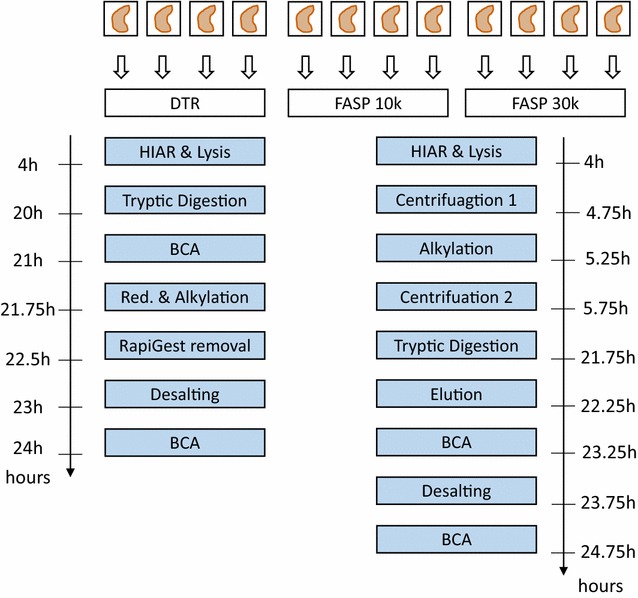
Schematic Workflow for the DTR versus FASP comparison. Four single, deparaffinized murine kidney FFPE tissues were separately prepared with each of the three sample preparation protocol. For the DTR protocol, a buffer containing 0.1% Rapigest in 0.1 M HEPES pH 8 and 1 mM DTT was used for heat-induced antigen retrieval (HIAR) and lysis of the tissue. As RapiGest is compatible with tryptic digestion, direct trypsinization is the key feature of the DTR protocol. RapiGest is later removed by acidifying the sample. Protein concentration is estimated to not overload the C18 stage tips during desalting step and later to inject the same amounts of peptides into the mass spectrometer. The FASP protocol makes use of a buffer containing 4% SDS, 0.1 M HEPES pH 7.5 and 0.05 M DTT. Before digestion the SDS is removed using centrifugal filter units with nominal molecular weight cut offs of either 10,000 or 30,000 Da. After digestion, the peptides are eluted from the filter units and desalted before mass-spectrometry analysis
