Abstract
Microglia are glial-immune cells that are essential for the function and survival of the central nervous system. Microglia not only protect neural tissues from immunological insults, but also play a critical role in neural development and repair. However, little is known about the biology of microglia in the cochlea, the auditory portion of the inner ear. In this study, we detected TMEM119+, CD11b+, CD45+ and Iba1+ populations of cells in the rat cochlea, particularly in Rosenthal’s canal, inner sulcus and stria vascularis. Next, we isolated and enriched the population of CD11b+ cells from the cochlea and immortalized these cells with the 12S E1A gene of adenovirus in a replication-incompetent retroviral vector to derive a novel microglial cell line, designated Mocha (microglia of the cochlea). The resulting Mocha cells express a number of markers consistent with microglia and respond to lipopolysaccharide (LPS) stimulation by upregulation of genes (Cox2, ICAM-1, Il6r, Ccl2, Il13Ra and Il15Ra) as well as releasing cytokines (IL-1beta, IL-12, IL-13 and RANTES). As evidence of microglial function, Mocha cells phagocytose fluorescent beads at 37 °C, but not at 4 °C. The expression pattern of microglial markers in Mocha cells suggests that immortalization leads to a more primitive phenotype, a common phenomenon in immortalized cell lines. In summary, Mocha cells display key characteristics of microglia and are now available as a useful model system for the study of cochlear microglial behavior, both in vitro and in vivo.
Introduction
Microglia are a unique lineage of mononuclear phagocytic cells that play an essential role in development, immunological defense and inflammatory processes in the central nervous system (review-Prinz and Priller, 2014). In development, microglia carry out synaptic pruning and remodeling (Paolicelli, et al., 2011) while also secreting brain-derived neurotrophic factor and other factors that modulate synaptic plasticity (Parkhurst, et al., 2013). In adult neural tissues, microglia are the first line of immunological defense, transitioning from a surveilling state to an activated state in response to conditions of cytotoxicity and cellular damage (Nimmerjahn, et al., 2005). The multifunctional behavior of microglia is very dependent on environmental cues and tissue location as well as complex interactions with surrounding cell types (Kabba, et al., 2017). Microglia have been described in the context of the brain, spinal cord (Galatro, et al., 2017), and retina (Arroba, et al., 2017), but little is known about the biology of microglial cells in the cochlea of the inner ear, a site vulnerable to damage by aging, noise and ototoxic compounds. Very recent studies have begun to address the role of Iba1+ microglia in response to noise exposure in the peripheral and central auditory system of the rat (Fuentes-Santamaria, et al., 2017), but much more characterization is needed. To address this gap in knowledge, we explored the possibility of harvesting and enriching microglia from the rat cochlea with the goal of immortalizing and cultivating them in culture as a novel microglial cell line.
Here, we demonstrate multiple microglial markers in the p3 rat cochlea, enrichment of CD11b+ cells, followed by the establishment and characterization of a novel immortalized microglial cell line. This cell line, designated Mocha (microglia of the cochlea), was immortalized using the growth-stimulating 12S gene of adenovirus in a replication-incompetent viral vector (Cone, et al., 1988). The resulting Mocha cells express markers associated with primary microglial cells and respond to pro-inflammatory lipopolysaccharide (LPS) stimulation by alterations in gene expression and cytokine/chemokine release consistent with inflammation. In addition, Mocha cells demonstrate the capacity to phagocytose fluorescent beads at physiological temperature, a hallmark of microglial function. In summary, Mocha cells provide a unique 12S E1A-immortalized microglial cell culture model of cochlear origin for evaluation of microglial behavior and function, both in vitro and in vivo, allowing for a better understanding of inflammatory processes in the cochlea related to health and disease.
METHODS
Cochlear explant culture
All animal studies were carried out with prior approval by the Institutional Animal Care and Use Committee of the University at Buffalo. The procedures for preparing rat cochlear organotypic cultures have been described previously (Ding, et al, 2002, 2011, 2012, 2014). Briefly, on postnatal day 3, rat pups were decapitated. The cochleae were then removed and placed in Hank’s balanced salt solution (1X GIBCO, 14175, Invitrogen, Carlsbad, CA). After removing the lateral wall, the whole basilar membrane containing the organ of Corti and spiral ganglion neurons were isolated, transferred onto a drop of 10 μl collagen gel matrix in a 35 mm culture dish. The collagen gel was prepared with a solution consisting of 15 μl of rat tail collagen (Type 1, BD Biosciences, 4236 Bedford, MA), 10×basal medium eagle (BME, Sigma B9638), and 2% sodium carbonate in a 9:1:1 ratio. The collagen gel was submerged in 1.3 ml of serum-free medium consisting of 2 g bovine serum albumin (BSA, Sigma A-4919), 2 ml serum-free supplement (Sigma I-1884), 4.8 ml of 20% glucose (Sigma G-2020), 0.4 ml penicillin G (Sigma P-3414), 2 ml of 200 mM glutamine (Sigma G-6392), and 190.8 ml of 1X BME (Sigma B-1522), and the cochlear explants were then incubated for 24 h (Forma Scientific 3029, 37°C in 5% CO2).
Microglia enrichment and cell culture
Rat cochleae (Charles River, Wilmington, MA) were harvested from postnatal day 3 Sprague-Dawley rats. To create the Mocha cell line, cochleae were mechanically triturated to promote a single-cell suspension. Immunomagnetic positive enrichment of cochlear-derived microglia was performed with the EasySep “Do It Yourself” Selection Kit (STEMCELL Technologies, Vancouver, BC Canada), coupled with an antibody against the microglia cell surface marker CD11b (Ab8879, Abcam, Cambridge, MA). Microglia-enriched cochlear cells were plated in 35 mm dishes in DMEM with 10% calf serum. After 48 hours, cells were incubated with a replication-incompetent 12S E1A-immortalizing retrovirus (Cone, et al. 1988) for two rounds of four hours each. These cell cultures were expanded for 4 days, followed by neomycin treatment for two weeks to select for cells expressing G418 resistance associated with the E1A immortalizing vector. Mocha cells were expanded and sent to IDEXX-RADIL testing labs (Columbia, MO) for authentication and short tandem repeat (STR) marker analysis.
Immunocytochemistry
The following primary antibodies were used for immunocytochemistry of cochlear explants and Mocha cells: rabbit anti-TMEM119 (1 μg/ml, NBP2-30551, Novus Biologicals, Littleton, CO), mouse anti-CD11b (2.7 ∝μg/ml, Ab8879, Abcam, Cambridge, MA), mouse anti-Iba (2 μg/ml, Santa Cruz Biotechnology), mouse anti-CD68 (5 μg/ml, MCA341GA, Bio-Rad, Hercules, CA), rabbit anti-CD45 (10 μg/ml, Ab10558, Abcam), mouse anti-E1A (2 μg/ml, Ab33183, Abcam), mouse anti-F4/80 (2 μg/ml, sc-377009, Santa Cruz, Santa Cruz, CA), rabbit anti-IL1-beta (5 μg/ml, Ab9722, Abcam), rabbit anti-SOX10 (1:100 dilution Abcam, Ab155279), CD40 (1:100, NB100-56127, Novus Biologicals,), beta-tubulin (10 ∝g/ml, Sigma, St. Louis, MO.). The secondary antibodies used for both cochlear explants and Mocha cells were: Dylight-488 anti-rabbit and Dylight-549 anti-mouse antibodies (5 μg/ml, Vector Laboratories, Burlingame, CA).
Cochlear explants were fixed for 2 hours in 10% phosphate buffered formalin, rinsed with 0.01 M PBS and then incubated at 4 °C overnight in primary antibody solution containing primary Ab, horse serum (10%), Triton X-100 (5%) and 0.01 M PBS. Following rinses with PBS, the tissues were then incubated at 4 °C overnight in a secondary antibody solution containing secondary Ab, horse serum (10%), Triton X-100 (5%) and 0.01 M PBS. Following a rinse in 0.01M PBS, the tissues were incubated with TO-PRO-3 (T3605 Life Technologies, Grand Island, NY) to label cell nuclei just prior to visualization. Tissues were mounted on glass slides in glycerin, coverslipped, and viewed with a confocal microscope (Zeiss LSM-510) with appropriate fluorescence filters.
Mocha cell cultures were grown on glass coverslips and stained for a variety of microglial and non-microglial markers. Briefly, cells were fixed on coverslips with either 4% paraformaldehyde or cytospin solution (72% isopropyl alcohol, 19% acetone, 7.6% glycerol) for ten minutes and stored in PBS buffer (PBS; 0.15 M NaCl, 8 mM Na2HPO4, 2.6 mM KCl, 1.5 mM KH2PO4) at 4 °C until staining. Cells on coverslips were permeabilized with PBS+0.05% Tween-20 for five minutes, followed by incubation in primary antibody or isotype control antibody (negative control) for one hour. Cells were rinsed in PBS, and incubated in fluorescent secondary antibody for 45 minutes at room temperature with anti-rabbit Dylight 488 or anti-mouse Dylight 555 (Vector Labs, Burlingame, CA) at 5 μg/ml in PBS with 0.05% Tween-20. Coverslips were mounted on slides with Vectashield mounting medium (Vector Labs) containing DAPI counterstain. Digital images were captured with a SONY ICX 285AL SPOT camera (Diagnostic Instruments, Sterling Heights, MI).
ELISA Analysis
In order to determine the secretion of cytokines and chemokines by resting and LPS-stimulated Mocha cells, we utilized a Multi-Analyte ELISArray from Qiagen (Germantown, MD; Cat no: MER-004A) to evaluate the presence of 12 cytokines and chemokines: IL1α, IL1β, IL2, IL4, IL5, IL10, IL12, IL13, IFN-γ, TNF-α, GM-CSF, and RANTES. Twenty-four hour conditioned media from Mocha cells and R28 cells (treated/not treated with 2 μg/ml LPS) were collected, concentrated from 7 ml to 0.7 ml using Amicon Ultra centrifugal filters (3000 NMWL) at 3000 g for 50 minutes. Non-conditioned medium was used as a negative control.
Gene Array
We designed a custom PCR gene array (SA Biosciences, Germantown, MD) to investigate expression of genes anticipated to be present/absent in microglia. In addition, the array contained primers for housekeeping genes (LDHA, ACTB, B2M, HPRT1 and RPLP1) to facilitate normalization, genomic DNA primer to detect genomic DNA contamination, transcription controls and positive PCR controls to test the efficiency of cDNA conversion as well as the PCR reaction. The PCR reaction was carried out using SYBR Green fluorescence (SABiosciences) technology measured by a Bio- Rad MyiQ Single Color Real Time PCR System. Cycle threshold (CT) values were determined for each gene of the array.
Phagocytosis
As a functional assay to measure phagocytosis, we added a 1:1000 dilution of 1.0 micron (non-opsinized) fluorescent beads comprised of carboxylate-modified polystyrene (excitation 470 nm; emission 505 nm) (Sigma, St. Louis, MO) to cultures of Mocha cells or R28 cells in standard culture medium. Cells were incubated with beads for up to 2 hours at 4 °C and 37 °C and rinsed with PBS. We captured still images using a Spot camera (Spot Imaging Solutions, Sterling MI). Multifocal images of ingested beads were obtained after incubating Mocha cells with fluorescent polystyrene beads for 2 hours, followed by a brief incubation with 10 μg/ml Hoechst 33342 nuclear stain (Invitrogen, Eugene Oregon). A Zeiss Axio Observer microscope (Carl Zeiss Microscopy, Peabody, MA) equipped with a Zeiss Apotome structured-illumination system and deconvolution software were used for obtaining high-resolution optical slices for 3-D reconstruction of fluorescent beads within cells.
RESULTS
Microglial markers expressed in rat cochlea
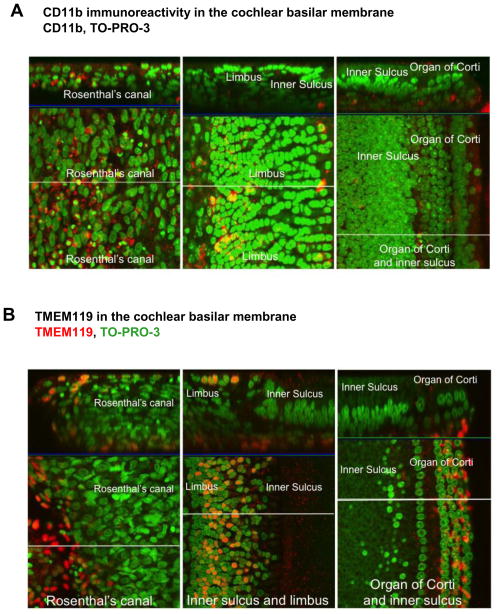
We examined p3 rat cochlea for immunoreactivity to microglial markers CD11b, Iba1 and TMEM119. We chose p3 rat cochlea specifically to match the tissue from which microglial cells were harvested for immortalization. In Figure 1A, CD11b immunolabeling was seen predominantly in Rosenthal’s canal, with somewhat fewer CD11b+ cells in the limbus, inner sulcus and organ of Corti. In Figure 1B, we observed TMEM119, a highly specific microglial marker (Satoh, et al., 2016) on numerous cells including the peripheral axon of spiral ganglion neurons, the limbus region and the organ of Corti. TMEM119+ and CD11b+ cells were also seen in the stria vascularis (Figure 1C). Double immunolabeling with TMEM119 and Iba1 revealed co-localization of immunopositive cells in Rosenthal’s canal and inner sulcus (Figure 1D). Based on microglia immunoreactivity and immune-colocalization in the cochlea, we utilized CD11b, a cell surface marker of microglia, to perform cell separation and enrichment of CD11b-positive cells in order to develop a novel cell line derived from cochlear microglia.
Figure 1. Microglial markers expressed in rat cochlea.
P3 rat cochlea were stained for microglial markers and viewed by confocal microscopy. (A) CD11b (red) immunoreactivity in the cochlear basilar membrane, limbus and Rosenthal’s canal. Green= nuclei. (B) TMEM119 (red) immunoreactivity in the cochlear basilar membrane, limbus and Rosenthal’s canal. Green = nuclei. (C) TMEM119 and CD11b immunoreactivity (red) in the stria vascularis. Green=nuclei. (D) Co-localization of TMEM119 and Iba1 in Rosenthal’s canal and inner sulcus. Blue = nuclei. Scale bars = 10 microns. Z axes are indicated in the top panels.
Microglial genes expressed by Mocha cells
Once the CD11b-enriched cells from cochlea were immortalized with the 12SE1A gene of adenovirus and survived G418 selection, we designed a custom gene array to determine the expression of genes typically expressed by microglia. Table 1 lists these genes that were expressed above background levels, after normalization with housekeeping genes, including TMEM119, IGF-1, CD68, TNFR1, CD54 (ICAM-1), CD40, CD74, Ccl2, B7-1, IFNgR, Sgpp1, IL1R1, IL13RA1, IL15RA, CCR5, Cox2, P2Y12, Il6R and MAP2. The elevated expression of these microglia-associated genes provides evidence of their microglia-like phenotype.
Table 1. Gene array analysis of Mocha cells.
A custom PCR gene array was used to analyze gene expression in Mocha cells. The abundance of inflammation-associated genes is presented relative to actin expression.
| S.No | Gene Sym | Δ Ct |
|---|---|---|
| 1 | Sgpp1 | 7.86 |
| 2 | CD54 | 8.49 |
| 3 | CD45 | 8.58 |
| 4 | TNFR1 | 8.71 |
| 5 | IGF-1 | 8.86 |
| 6 | B7-1 | 9.31 |
| 7 | Ifngr | 9.41 |
| 8 | IL1R1 | 10.44 |
| 9 | MAP2 | 10.76 |
| 10 | Cox2 | 10.91 |
| 11 | IL13RA1 | 11.33 |
| 12 | IL15RA | 12.98 |
| 13 | Tmem119 | 13.16 |
| 14 | Ccl2 | 14.24 |
| 15 | Il6r | 14.37 |
| 16 | CCR5 | 14.97 |
| 17 | CD68 | 15.57 |
| 18 | CD40 | 16.04 |
| 19 | P2Y12 | 16.35 |
| 20 | Cd74 | 17.19 |
| 21 | Il6 | 17.77 |
| 22 | IbA1 | 18.57 |
| 23 | GFAP | 18.70 |
| 24 | TNFR2 | 18.86 |
| 25 | TNFα | 19.86 |
| 26 | Rbfox3 | 20.26 |
Immunoreactivity of microglial markers in the Mocha cell line
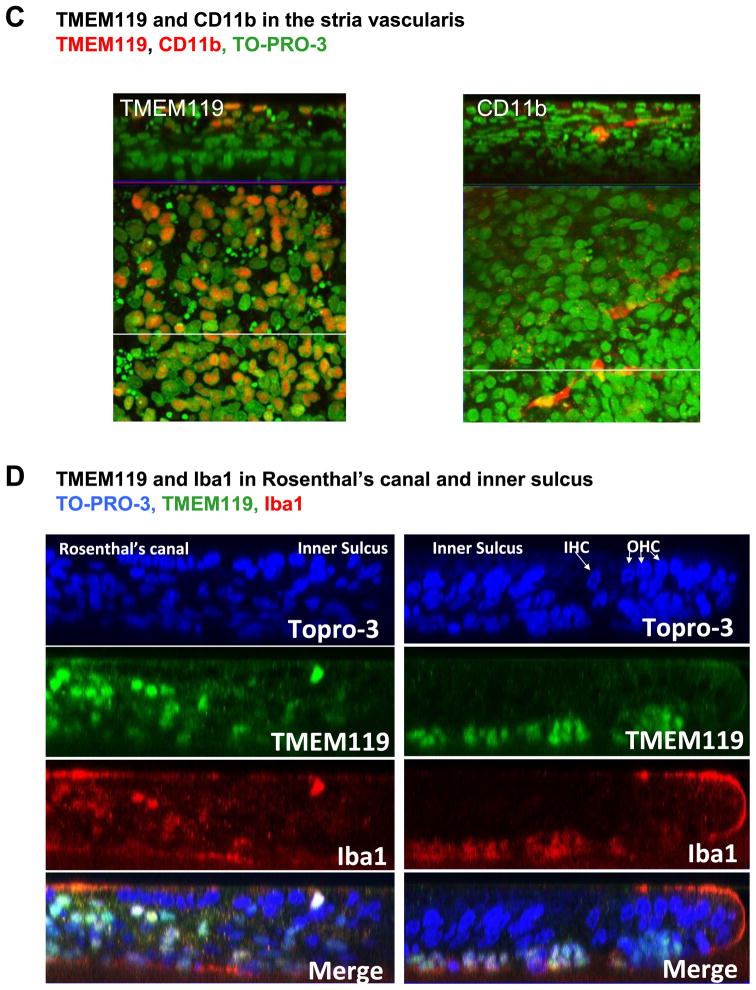
To validate gene array results with expression at the protein level, we carried out immunocytochemical analysis of Mocha cells to detect microglial marker immunoreactivity on a cell-by-cell basis. Figure 2 shows that Mocha cells were immunoreactive to a number of microglia markers, including CD68, Sox10, CD11b, CD40, Iba1, TMEM119, IL-1beta, CD45 and F4/80, as well as the 12SE1A protein used for immortalization. Mocha cells were immunonegative, as anticipated, for neuronal beta-tubulin.
Figure 2. Immunoreactivity of microglial markers in the Mocha cell line.
Mocha cells were immunostained as described in Methods. Immunoreactivity was seen for the 12S E1A immortalizing gene, CD68, Sox10, CD11b, CD40, Iba1, TMEM119, IL-1beta, CD45 and F4/80. Arrows indicate examples of immunoreactive cells. Neuronal beta tubulin and negative control panels show only blue nuclei detected by DAPI. Scale bar = 10 microns.
LPS activates Mocha cells and stimulates secretion of cytokines
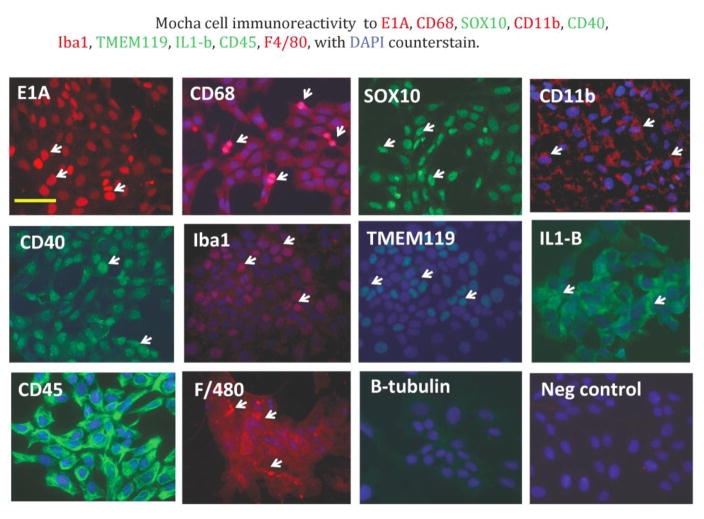
LPS is an activator of microglial cells, with known effects on gene expression and cellular activity. We treated Mocha cells with LPS for 24 hours to elicit a response, both in terms of gene expression and secretion of specific cytokines and chemokines. In Table 2, gene array analysis of Mocha cells revealed several genes responded to LPS treatment by increased expression levels, particularly Ccl2 (30-fold), Cox2 (2.9-fold), CD54 (3.3-fold) and IL6R (2.4-fold). In contrast, MAP2 decreased to 0.587 of the pre-LPS level.
Table 2. LPS activation of Mocha cells modulates expression of inflammatory genes.
A comparison of LPS-treated and untreated cells reveals changes in inflammatory gene expression. The original gene array from Table 1 was used and significant changes are presented here.
| Gene | Fold change | p value |
|---|---|---|
| Ccl2 | 30.015 | 0.000001 |
| CD54 | 3.2949 | 0.000001 |
| Cox2 | 2.9873 | 0.000851 |
| Il6r | 2.3725 | 0.000003 |
| IL13RA1 | 1.6595 | 0.025232 |
| IL15RA | 1.6634 | 0.00416 |
| MAP2 | 0.587 | 0.000001 |
We treated Mocha cells and R28 cells (as a control) with LPS for 24 hours to elicit the secretion of specific cytokines and chemokines expressed by microglia, but presumably not by R28 retinal precursor cells. In Figure 3, ELISA analysis of concentrated conditioned medium from Mocha cells showed statistically significant LPS enhanced secretion of IL1-beta, IL-12, IL-13 and RANTES. In contrast, LPS-treatment of R28 retinal precursor cells did not result in detectable secretion of IL1-beta, IL-2 or IL-13, while a low level of RANTES secreted under control conditions diminished upon LPS treatment (not shown).
Figure 3. LPS stimulates secretion of cytokines in Mocha cells.
(A) LPS treatment of Mocha cells promotes secretion of IL1-beta, IL-12, IL-13 and RANTES. (B) A complete list of cytokines measured with and without LPS treatment. Mean values are presented in pg/ml, +/− standard deviation.
Mocha cells phagocytose fluorescent beads
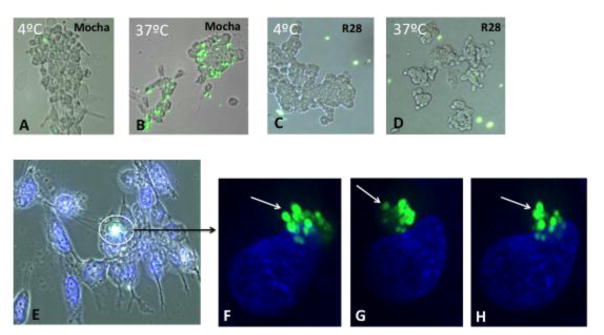
Phagocytosis is a hallmark of microglial cell behavior as scavengers of microorganisms and debris. We designed an experiment to evaluate phagocytosis by Mocha cells and R28 cells with the use of 1.0 micron fluorescent carboxylate-modified polystyrene latex beads. In the first experiment, Mocha cells and R28 cells were grown on glass coverslips and incubated with fluorescent beads at 4 °C and 37 °C, respectively. We used non-opsonized beads so as not to extrinsically promote cell-bead interactions. After one hour, we observed many fluorescent beads associated with Mocha cells at 37 °C (Figure 4B), with very few at 4 °C, presumably due to decreased metabolic activity at the lower temperature (Figure 4A). In contrast, R28 cells did not appear to associate with the beads at either temperature (Figure 4C and D). Although we could see beads closely associated with Mocha cells, it was not entirely clear at this magnification whether the beads were actually internalized. In order to confirm internalization, we incubated Mocha cells with fluorescent beads at 37 °C for 2 hours and viewed them under light microscopy (Figure 4E) and then under high-resolution confocal microscopy with deconvolution 3-D analysis. In Figure 4F–G, fluorescent beads are clearly internalized by Mocha cells, as seen in three different axes of the same cell, with the nucleus as a reference point.
Figure 4. Mocha cells phagocytose fluorescent beads.
Mocha cells were incubated with a 1:1000 dilution of 1.0 micron fluorescent beads comprised of carboxylate-modified polystyrene (excitation 470 nm; emission 505 nm) for up to 2 hours at 4 °C (A) and 37 °C (B) and rinsed with PBS. Panels C and D show R28 retinal cells under the same conditions. (E) Light microscopic image of Mocha cells incubated with fluorescent beads at 37 °C and labeled with Hoechst 33342 nuclear stain. (F–H) Confocal 3D renderings of the image stack shown in panel E (3 image planes at three levels of the nucleus) The 3D views in F–H are of the same cells/beads, but just shown at different angles around the x, y and z axis (see arrow).
Mocha cell line authentication
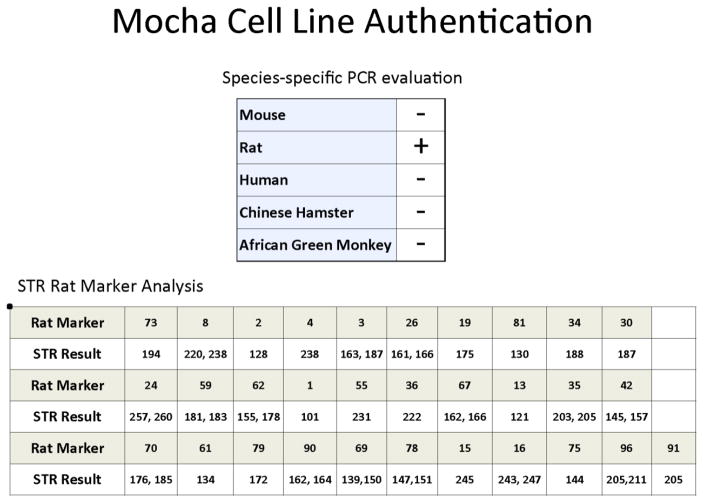
A frozen vial of Mocha cells at passage 5 was sent to IDEXX-RADIL for cell line authentication and short tandem repeat (STR) marker analysis, as shown in Figure 5. The Mocha cell line was confirmed to be of rat cell origin without other contaminating cell types. STR marker analysis is presented for future comparison and verification purposes.
Figure 5. Mocha cell line genotype.
Mocha cells were analyzed at p5 by IDEXX-RADIL for short tandem repeat (STR) markers and species of origin (rat).
DISCUSSION
In this study, we examined resident microglial cells present within the P3 rat cochlea, with potential implications for inflammatory processes within the cochlea related to development, damage and repair. Little is known about how the cellular debris from developing or damaged cochlea is removed. Because intense noise exposure, ototoxic drugs, aging and other ototraumatic events lead to necrotic and apoptotic cell death and subsequent release of inflammatory molecules and cellular debris that need to be phagocytized and removed, cochlear microglia likely serve as “first responders” in cases of chronic and acute cochlear pathology. Indeed, after an intense noise exposure that caused massive damage to the cochlea, we observed intense microglia immunolabeling around the degenerating auditory nerve fibers that project from the soma of spiral ganglion neurons in the cochlea to the cochlear nucleus in the brainstem (Baizer et al., 2015). Microglia upregulation and neural degeneration persisted for up to 9 months following the initial insult. As further evidence for trauma-mediated microglial activation in the cochlea, an increase in Iba1+ cells co-localized with TNF-alpha and IL1-beta has been reported in the spiral ganglion of noise-exposed rats (Fuentes-Santamaria, et al., 2017). These findings support the hypothesis that microglia may be an important source of pro-inflammatory cytokines, such as IL-1beta, IL-6 and TNF-alpha produced in response to noise damage in the cochlea (Fujioka, et al. 2006).
From CD11b+ enriched cells of the cochlea, we have developed a unique microglial precursor cell line called Mocha (microglia of the cochlea). Mocha cells express a number of known microglial markers, including TMEM119, Iba1, CD45, CD68 and Cox2. Mocha cells also express the 12S E1A immortalizing gene of adenovirus, as well as an accompanying neomycin (G418) resistance gene (Cone et. al, 1988). We have demonstrated physiological responses of Mocha cells consistent with microglial cell behavior. LPS stimulation of Mocha cells leads to increased expression levels of Cox2, CD54 (ICAM-1), Il6r, Ccl2, Il13Ra and Il15Ra, as well as increased secretion of IL1-beta, IL-12, IL-13 and RANTES; these cytokines and chemokines involved in inflammation were not secreted by R28 retinal cells in response to LPS treatment. These results suggest future studies in which these microglia-secreted cytokines and chemokines could be applied to the cochlea in vivo or in vivo to determine their functional and histological consequences.
Mocha cells are able to phagocytose fluorescent beads, a cellular function attributed to microglia. Future in vitro studies could be carried out to determine if Mocha cells inject the cellular debris released by damaged hair cells, neurons and supporting cells in cochlear organotypic cultures. Phagocytosis is more pronounced at 37 °C compared with 4 °C, an indication of increased cellular activity at physiological temperature.
It is currently unclear whether microglia derived from different species or different portions of the central nervous system share identical or divergent properties. Mocha cells are different from existing microglial cell lines, such as the mouse brain-derived SIM-A9 (Nagamoto-Combs, et al., 2014), N9 (Righi, et al., 1989), and BV2 cell lines (Blasi, et al., 1990) as well as the human HMO6 cell line (Nagai, et al., 2001). Mocha cells are derived from the rat cochlea and are immortalized with the 12S E1A gene using a replication-incompetent retroviral vector, which allows for in vivo studies without the confounding issue of host retroviral infection. The 12S E1A gene differs from the oncogenes used to immortalize the brain-derived murine microglial cell lines in that the 12S E1A gene is growth-promoting, but not tumorigenic by itself (Cone, et al., 1988). There are some differences in gene expression patterns between the microglial cell lines, but all are phagocytic and respond to activation by secretion of cytokines (Stansley, et al., 2012).
The Mocha microglial cell line has unique properties, due in part to the expression of 12S E1A, not found in other microglial cell lines. Immortalization is known to affect growth rate as well as expression of some genes associated with mature cell types. Proliferation can lead to a precursor-like state, which may account for the presence of MAP2, a neuronal protein that can be expressed by microglia under neuronal differentiating conditions as a function of plasticity (Matsuda, et al., 2008). Interestingly, MAP2 was downregulated in Mocha cells upon LPS activation, as shown in Table 2.
There were some minor differences in expression of microglial markers CD11b and IL-1beta across our detection platforms. Mocha cells displayed immunoreactivity to CD11b and IL-1beta and also secreted IL1-beta but did not display these markers in the gene array. This may be explained by differences in the specific probe used for the gene array vs. the epitope used for immunocytochemistry and ELISA, or the possibility of regulation of gene expression at the protein level, due in part to growth stimulation by expression of 12S E1A. TMEM119, a recently identified and highly specific marker for microglia (Satoh, et al., 2016) was expressed at significant levels in all platforms, as was Iba1. As with most other immortalized cell lines, Mocha cells are not a perfect substitute for primary cells, but represent a powerful tool for drug screening, gene expression analysis and studies of microglial cell behavior. To place this in context, R28, a sister cell line to Mocha, is a rat retinal precursor cell line established over 20 years ago also using the same 12S E1A immortalization technique (Seigel, 1996). R28 cells, like Mocha cells, do not express all markers of the mature cell type (Seigel et al., 2004), but can respond to some conditions that promote differentiation (Sperling, et al, 2012). R28 cells have been utilized in hundreds of studies, both in vitro and in vivo as a model for cytotoxicity (Patil, et al., 2009), differentiation (Sperling et al., 2012), transplantation (Seigel, et al., 1998) and retinal cell behavior (Uddin, et al., 2016). We anticipate that Mocha cells will show similar utility in studies relating to the inner ear as an immortalized cell line. Factors secreted from Mocha cells in response to ototoxic drugs such as cisplatin could be evaluated to determine if these secreted factors are themselves toxic or promote repair, differentiation or survival. Drugs screens could be conducted with Mocha cells to identify compounds that suppress or promote proliferation or activation. Co-culture studies with other cochlear cell types could aid in elucidating the role of microglial cells in development and neuroprotection in the inner ear. Microglia appear to be involved in synaptic remodeling and factors secreted by microglia could be used to determine how they affect the afferent and efferent synapses expressed in the cochlea. These studies will lead to a better understanding of signaling pathways involved in microglial function that can aid in the development of targeted interventions for the inner ear to maintain and restore auditory function.
Highlights.
We present the new immortalized Mocha cell line derived from p3 rat cochlea.
Mocha cells express key microglial markers and phagocytose fluorescent beads.
LPS activation of Mocha cells causes changes in gene expression and cytokine release.
Mocha cells are a novel model system for the study of microglia from the cochlea.
Acknowledgments
This work was supported in part by grants from the National Institute for Occupational Safety and Health, R01 OH010235 and the National Institutes of Health, NIDCD R01DC011808, R01DC014452 and R01DC014693, China Scholarship Council, No 201606095026 and The National Nature Science Foundation of China (NSFC No. 81520108015). We thank Dr. Wade Sigurdson, director of the Confocal Microscope and Flow Cytometry Core Facility at the University at Buffalo, for assistance with confocal imaging and analysis. We thank Dr. Steven Khoury for technical advice regarding ELISA sample preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arroba AI, Valverde ÁM. Modulation of microglia in the retina: new insights into diabetic retinopathy. Acta Diabetol. 2017 Jun;54(6):527–533. doi: 10.1007/s00592-017-0984-z. Epub 2017 Mar 27. [DOI] [PubMed] [Google Scholar]
- Baizer JS, Wong KM, Manohar S, Hayes SH, Ding D, Dingman R, Salvi RJ. Effects of acoustic trauma on the auditory system of the rat: the role of microglia. Neuroscience. 2015 Sep 10;303:299–311. doi: 10.1016/j.neuroscience.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett ML, Bennett FC, Liddelow SA, Ajamid B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adila A, Tucker A, Weissman IL, Chang EF, Li G, Grant GA, Hayden Gephart MG, Barres BA. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A. 2016 Mar 22;113(12):E1738–46. doi: 10.1073/pnas.1525528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi E, Barluzi R, Bocchini V, Mazolla R, Bistoni F. Immortalization of murine microglia cells by a v-raf/v-myc carrying retrovirus. J Neuroimmun. 1990;27:229–237. doi: 10.1016/0165-5728(90)90073-V. [DOI] [PubMed] [Google Scholar]
- Cherry JD, Olschowka JA, O’Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation. 2014 Jun 3;11:98. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD, Grodzicker T, Jaramillo M. A retrovirus expressing the 12S adenoviral E1A gene product can immortalize epithelial cells from a broad range of rat tissues. Mol Cell Biol. 1988 Mar 19;8(3):1036–1044. doi: 10.1128/mcb.8.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Stracher A, Salvi RJ. Leupeptin protects cochlear and vestibular hair cells from gentamicin ototoxicity. Hear Res. 2002;164:115–126. doi: 10.1016/S0378-5955(01)00417-8. doi: 10.1016/S0378-5955(01)00417-8. [DOI] [PubMed] [Google Scholar]
- Ding D, He J, Allman BL, Yu D, Jiang H, Seigel GM, Salvi RJ. Cisplatin ototoxicity in rat cochlear organotypic cultures. Hear Res. 2011;282:196–203. doi: 10.1016/j.heares.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Jiang H, Fu Y, Salvi R, Someya S, Tanokura M. Ototoxic effects of carboplatin in cochlear organotypic cultures in chinchillas and rats. J of Otol. 2012;7:92–101. doi: 10.1016/S1672-2930(12)50023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Qi W, Yu D, Jiang H, Han C, Kim MJ, Katsuno K, Hsieh YH, Miyakawa T, Salvi R, Tanokura M, Someya S. Addition of Exogenous NAD+ Prevents Mefloquine-Induced Neuroaxonal and Hair Cell Degeneration through Reduction of Caspase-3-Mediated Apoptosis in Cochlear Organotypic Cultures. PLoS ONE. 2013;8(11):e79817. doi: 10.1371/journal.pone.0079817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Salvi R, Roth J. Cellular localization and developmental changes of Zip8, Zip14 and transferrin receptor 1 in the inner ear of rats. Biometals. 2014;27:731–744. doi: 10.1007/s10534-014-9765-0. [DOI] [PubMed] [Google Scholar]
- Fuentes-Santamaria V, Alvarado JC, Melgar-Rojas P, Gabaldon-Ull MC, Miller JM, Juiz JM. The Role of Glia in the Peripheral and Central Auditory System Following Noise Overexposure: Contribution of TNF-alpha and IL-1beta to the Pathogenesis of Hearing Loss. Front Neuroanat. 2017;11:9. doi: 10.3389/fnana.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka M, Kanzaki S, Okano HJ, Masuda M, Ogawa K, Okano H. Proinflammatory cytokines expression in noise-induced damaged cochlea. J Neurosci Res. 2006 Mar;83(4):575–83. doi: 10.1002/jnr.20764. [DOI] [PubMed] [Google Scholar]
- Galatro TF, Vainchtein ID, Brouwer N, Boddeke EW, Eggen BJ. Isolation of Microglia and Immune Infiltrates from Mouse and Primate Central Nervous System. Methods Mol Biol. 2017;1559:333–342. doi: 10.1007/978-1-4939-6786-5_23. [DOI] [PubMed] [Google Scholar]
- Kabba JA, Xu Y, Christian H, Ruan W, Chenai K, Xiang Y, Zhang L, Saavedra JM, Pang T. Microglia: housekeeper of the central nervous system. Cell Mol Neurobiol. 2017 doi: 10.1007/s10571-017-0504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamoto-Combs Kumi, Kulas Joshua, Combs Colin K. A novel cell line from spontaneously immortalized murine microglia. J Neurosci Methods. 2014;233:187–98. doi: 10.1016/j.jneumeth.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Niidome T, Nonaka H, Goto Y, Fujimura K, Kato M, Nakanishi M, Akaike A, Kihara T, Sugimoto H. Microtubule-associated protein 2-positive cells derived from microglia possess properties of functional neurons. Biochem Biophys Res Commun. 2008;368(4):971–6. doi: 10.1016/j.bbrc.2008.02.038. Epub 2008 Feb 20. [DOI] [PubMed] [Google Scholar]
- McCarthy RC, Lu DY, Alkhateeb A, Gardeck AM, Lee CH, Wessling-Resnick M. Characterization of a novel adult murine immortalized microglial cell line and its activation by amyloid-beta. Journal of Neuroinflammation. 2016;13:21. doi: 10.1186/s12974-016-0484-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai A, Nakagawa E, Hatori K, Choi HB, McLarnon JG, Lee MA, Kim SU. Generation and characterization of immortalized human microglial cell lines: expression of cytokines and chemokines. Neurobiol Dis. 2001;8:1057–1068. doi: 10.1006/nbdi.2001.0437. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- O’Malley JT, Nadol JB, Jr, McKenna MJ. Anti CD163+, Iba1+, and CD68+ cells in the adult human inner ear – Normal distribution of an unappreciated class of macrophages/microglia and implications for inflammatory otopathology in humans. Otol Neurotol. 2016;37(1):99–108. doi: 10.1097/MAO.0000000000000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano T, Nakagawa T, Kita T, Kada S, Yoshimoto M, Nakahata T, Ito J. Bone marrow-derived cells expressing Iba1 are constitutively present as resident tissue macrophages in the mouse cochlea. J Neurosci Res. 2008;86:1758–1767. doi: 10.1002/jnr.21625. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011 Sep 9;333(6048):1456–8. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, Lafaille JJ, Hempstead BL, Littman DL, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil AJ, Gramajo AL, Sharma A, Chwa M, Seigel GM, Kuppermann BD, Kenney MC. Effects of benzo(e)pyrene on the retinal neurosensory cells and human microvascular endothelial cells in vitro. Curr Eye Res. 2009 Aug;34(8):672–82. doi: 10.1080/02713680903015892. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nature Reviews Neuroscience. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- Righi M, Mori L, De Libero G, Sironi M, Biondi A, Mantovani A, Donini S, Ricciardi-Castagnoli P. Monokine production by microglial cell clones. Eur J Immunol. 1989;19:1443–1448. doi: 10.1002/eji.1830190815. [DOI] [PubMed] [Google Scholar]
- Satoh J-i, Kino Y, Asahina N, Takitani M, Miyoshi J, Ishida T, Saito Y. TMEM119 marks a subset of microglia in the human brain. Neuropathology. 2016;36:39–49. doi: 10.1111/neup.12235. [DOI] [PubMed] [Google Scholar]
- Seigel GM. Establishment of an E1A-immortalized rat retinal cell culture. In Vitro Cell Devel Biol. 1996;32:66–68. doi: 10.1007/BF02723034. [DOI] [PubMed] [Google Scholar]
- Seigel GM, Sun W, Wang J, Hershberger DH, Campbell LM, Salvi RJ. Neuronal gene expression and function in the growth-stimulated R28 retinal precursor cell line. Curr Eye Res. 2004;28(4):257–269. doi: 10.1076/ceyr.28.4.257.27831. [DOI] [PubMed] [Google Scholar]
- Seigel GM, Takahashi M, Adamus G, McDaniel T. Intraocular transplantation of E1A-immortalized retinal precursor cells. Cell Transplant. 1998;7(6):559–566. doi: 10.1177/096368979800700606. [DOI] [PubMed] [Google Scholar]
- Sperling LE, Klaczinski J, Schütz C, Rudolph L, Layer PG. Mouse acetylcholinesterase enhances neurite outgrowth of rat R28 cells through interaction with laminin-1. PLoS One. 2012;7(5):e36683. doi: 10.1371/journal.pone.0036683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansley B, Post J, Hensley K. A comparative review of cell culture systems for the study of microglial biology in Alzheimer’s disease. J Neuroinflammation. 2012;9:115. doi: 10.1186/1742-2094-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin MI, Evans SM, Craft JR, Capozzi ME, McCollum GW, Yang R, Marnett LJ, Uddin MJ, Jayagopal A, Penn JS. In Vivo Imaging of Retinal Hypoxia in a Model of Oxygen-Induced Retinopathy. Sci Rep. 2016;6:31011. doi: 10.1038/srep31011. [DOI] [PMC free article] [PubMed] [Google Scholar]