Key Points
Question
Are patients with a patent foramen ovale at increased risk of perioperative ischemic stroke?
Findings
In this retrospective cohort study that included 182 393 adults who underwent noncardiac surgery, preoperatively diagnosed patent foramen ovale (PFO) was significantly associated with an increased risk of ischemic stroke within 30 days after surgery (3.2% vs 0.5%).
Meaning
Preoperatively diagnosed PFO may be associated with increased risk of perioperative stroke following noncardiac surgery.
Abstract
Importance
Perioperative stroke is a major complication for patients undergoing surgery. Patent foramen ovale (PFO) represents a possible anatomical link between venous thrombosis and stroke.
Objective
To determine whether a preoperatively diagnosed PFO is associated with increased risk of perioperative ischemic stroke.
Design, Setting, and Participants
Retrospective cohort study from Massachusetts General Hospital and 2 affiliated community hospitals between January 1, 2007, and December 31, 2015. Participants were 182 393 consecutive adults undergoing noncardiac surgery with general anesthesia.
Exposures
Preoperatively diagnosed PFO.
Main Outcomes and Measures
Perioperative ischemic stroke occurring within 30 days of surgery; stroke subtype by Oxfordshire Community Stroke Project classification and stroke severity by National Institute of Health Stroke Scale (NIHSS).
Results
Among the 150 198 patient cases analyzed (median [SD] age, 55 [16] years), 1540 (1.0%) had a diagnosis of PFO before surgery. A total of 850 (0.6%) ischemic strokes occurred within 30 days of surgery (49 [3.2%] among patients with PFO and 801 [0.5%] among patients without PFO). In adjusted analyses, patients with PFO had an increased risk of ischemic stroke compared with patients without PFO (odds ratio, 2.66 [95% CI, 1.96-3.63]; P < .001). The estimated risks of stroke were 5.9 for every 1000 patients with PFO and 2.2 for every 1000 patients without PFO (adjusted absolute risk difference, 0.4% [95% CI, 0.2%-0.6%). Patients with PFO also had an increased risk of large vessel territory stroke (relative risk ratio, 3.14 [95% CI, 2.21-4.48]; P < .001) and a more severe stroke-related neurologic deficit measured by NIHSS (median, 4 [interquartile range {IQR}, 2-10] vs median, 3 [IQR, 1-6] for those without PFO; P = .02).
Conclusions and Relevance
Among adult patients undergoing noncardiac surgery at 3 hospitals, having a preoperatively diagnosed PFO was significantly associated with increased risk of perioperative ischemic stroke within 30 days after surgery. Further research is needed to confirm these findings and to determine whether interventions would decrease this risk.
This cohort study compares risk of ischemic stroke within 30 days postsurgery among adult noncardiac surgery patients with patent foramen ovale (PFO) vs without PFO.
Introduction
The annual global surgical volume has been increasing, with an estimated 312 million surgical procedures taking place worldwide in 2012. Perioperative stroke is a major postoperative complication, with significant implications on postoperative morbidity, discharge disposition, and 30-day mortality. With a reported incidence ranging from 0.2% to 9.7% for patients undergoing different surgeries, and possibly an even higher incidence of covert strokes, the estimated annual global burden of perioperative stroke could exceed 1 million.
There has been an increasing interest in the association between a patent foramen ovale (PFO) and stroke. The autopsy prevalence of PFO in a general population is approximately 27.3%. Although the majority of the population with PFO remains undiagnosed, the significance of PFO in causing stroke in specific cohorts is increasingly clear. The current knowledge regarding the PFO-stroke relationship has mainly come from moderately sized case-control studies and meta-analyses in subgroups of young patients with cryptogenic stroke, and the effects of preoperatively diagnosed PFO on perioperative stroke are unknown.
During and after surgery and anesthesia, patients with PFO are exposed to various physiological threats that increase the vulnerability to stroke due to paradoxical embolism, including hemodynamic changes that increase right-to-left shunting and also hypercoagulability and formation of venous thromboemboli. This study tested the hypothesis that a preoperatively diagnosed PFO is associated with increased risk of perioperative ischemic stroke among patients undergoing noncardiac surgery.
Methods
Study Population and Design
Data from all patients who underwent surgery between January 1, 2007, and December 31, 2015, at Massachusetts General Hospital and 2 affiliated community hospitals (Mass General West, in Waltham, and Mass General/North Shore Center for Outpatient Care, in Danvers) were reviewed. The study was approved by the Partners institutional review board (protocol number 2017P000260 in Supplement 1), and a waiver of informed consent was granted (for details regarding this registry, see section 2.2 in Supplement 2).
All adult patients (aged≥18 years) who underwent surgery under general anesthesia and mechanical ventilation and were extubated at the end of the procedure were included. Exclusion criteria were patients with American Society of Anesthesiologists (ASA) physical status classification of VI (patients with brain death), and patients undergoing cardiac or pediatric surgeries.
Definitions of Exposure and Outcomes
The presence of a preoperatively diagnosed PFO was determined using the International Classification of Diseases, Ninth Revision and Tenth Revision (ICD-9 and ICD-10) diagnostic codes 745.5 and Q21.1 (eTable 1 in Supplement 2). Patients without a billing diagnosis of PFO were considered not exposed. Patients with a history of PFO closure were classified into the without PFO group.
The primary outcome was perioperative ischemic stroke within 30 days of surgery based on ICD-9 and ICD-10 diagnostic codes and confirmed by medical record review. Review of all patients with a diagnostic coding of ischemic stroke within 30 days after surgery were conducted by reviewers blinded to PFO status with a standardized methodology in reviewing patient notes, neurologist assessments, and findings from radiological studies such as computed tomography or magnetic resonance imaging of the brain. Details of the stroke, including the date and timing, the stroke subtype by Oxford Community Stroke Project classification, and the stroke-related neurological deficit measured by National Institute of Health Stroke Scale (NIHSS) were obtained retrospectively in the following order of priority: as scored by neurologists in neurology notes, as recorded on reports of radiological studies, and as abstracted from records. When the information was abstracted from records, the raters were blinded to PFO status. The secondary outcomes included 30-day hospital readmission, defined as an inpatient readmission to a hospital in the Partners health care network, and 30-day mortality.
Statistical Analyses
Based on the observation that patients who received a PFO occlusion are 50% less likely to have a recurrent nonfatal ischemic stroke than patients in the medical management group, we defined prior to conducting the analysis, an odds ratio (OR) of 2.0 as a clinically meaningful association between PFO and perioperative ischemic stroke. The sample size had 94.3% power to detect a difference in risk of stroke between patient groups, assuming an observed PFO rate of 1.0%, a perioperative ischemic stroke event rate of 0.5%, and a 1-sided α level of .025.
All analyses were performed retrospectively with prespecified end points and statistical methods. Unadjusted analyses were made using χ2 tests for categorical variables and the Student t test or Wilcoxon rank-sum test for continuous variables. Multivariable logistic regression was performed to evaluate the relationship between having a PFO and perioperative ischemic stroke, controlling for confounding variables selected a priori based on data in the published literature and biological plausibility. The confounding variables included baseline patient characteristics such as age, sex, body mass index, ASA physical status classification, and Charlson comorbidity index; coexisting conditions such as history of cigarette smoking, hypertension, diabetes, dyslipidemia, coronary artery disease, myocardial infarction, congestive heart failure, pulmonary edema, pulmonary hypertension, cardiomyopathy, congenital heart disease, atrial fibrillation, valvular heart disease, chronic obstructive pulmonary disease, migraine, chronic kidney disease, hypercoagulable state, deep vein thrombosis, pulmonary embolism, and systemic embolic phenomenon; prescription within 28 days before surgery of β-blockers, statins, antiplatelet agents, and anticoagulants. Factors relating to surgery, including emergency surgery status, inpatient surgery, high-risk surgical service, duration of surgery, intraoperative hypotensive minutes, intraoperative dose of vasopressors, intraoperative fluid volumes, requirement for packed red blood cells transfusion, and work relative value units—a marker of procedural complexity, were also adjusted for. The primary regression model for perioperative ischemic stroke was conducted using forced variable entry and evaluated to ensure that the estimates could be interpreted conventionally. Model discrimination was assessed through the concordance C statistic. Model calibration was assessed by the Hosmer-Lemeshow test and a reliability plot, which analyzed the agreement between the observed and estimated outcomes. Model resolution was assessed by plotting a histogram of the log of the estimated values. All continuous variables in the primary regression model were tested for linearity; nonlinear variables were categorized in quintiles.
The association between PFO and ischemic stroke subtype, using the Oxfordshire Community Stroke Project classification, was analyzed with a multinomial logistic regression model and reported as relative risk ratios (RRs).
Sensitivity Analyses
To address potential biases in the diagnosis of PFO that were not considered in the multivariable confounder model, a logistic regression analysis was used to create a propensity score that estimated the likelihood of diagnosed PFO, based on coexisting medical conditions that may predispose a referral for echocardiography studies. Propensity score matching was performed using a 1:5 matching ratio with nearest neighbor and sampling without replacement with a caliper of 0.20. The association of PFO with perioperative ischemic stroke was tested in the 1:5 propensity score–matched cohort.
To further control for unmeasured differences that biased the referral for evaluation by echocardiogram, the primary analysis was repeated in a subgroup including only patients with a history of a documented echocardiogram in the same institution prior to the index surgery. Details were obtained regarding the mode of echocardiography (transthoracic vs transesophageal [TEE]), as defined by Current Procedural Terminology codes, and whether injection of agitated saline contrast was performed. Sensitivity analyses were repeated in these subgroups to examine the effects of residual confounding. Since Current Procedural Terminology codes cannot be used to identify contrast echocardiography studies, they were identified by applying a text search function to all retrieved echocardiography reports. This text search method was validated by medical records review of 200 randomly selected patients (100 with and 100 without agitated saline testing).
To examine whether the PFO-attributable risk of stroke differs for patients at different stroke risks, a probability score for the baseline risk of perioperative ischemic stroke independent of PFO diagnosis was created, based on comorbid conditions and surgical factors that are risk factors for stroke, and the PFO-stroke association was reexamined for heterogeneity across this baseline risk. Effect modification on the association between PFO and perioperative ischemic stroke by patients’ baseline stroke risk was tested by introducing an interaction term to the multivariable regression model.
Although the medical records–reviewed outcome of perioperative ischemic stroke was used in the primary analysis, the effect estimate when the outcome variable was defined using the same process of classification as other variables, that is by ICD-9 and ICD-10 codes, was tested.
To better ensure that the observed association between PFO and stroke was not due to some underlying cause unrelated to the mechanistic hypothesis, falsification testing was performed with 3 postoperative outcomes: septic shock, wound complication, and peptic ulcer disease. These outcomes were selected based on a common contributing etiology of nonthrombotic tissue ischemia but unlikely to be causally related to the presence or absence of PFO. Further falsification testing in the complete patient cohort, as well as the cohort with history of echocardiography, was performed using an exposure variable that is theoretically not directly associated with increased stroke risk—tricuspid valve disorders.
The complete case method was adopted to address missing data in the primary statistical analysis. The analysis was repeated with the entire cohort using the technique of multiple imputations by chained equations.
Exploratory Analyses
The relationship between PFO and other perioperative complications of embolic etiology, such as acute limb ischemia and renal artery embolism, was explored.
Additional analyses have been conducted as described in Supplement 3. Because there were no adjustments for multiple comparisons, the prespecified secondary end points should be interpreted as exploratory. Data management and statistical analyses were performed using Stata software, version 13 and R Studio software, version 3.2.5. A 2-tailed P value of less than .05 was considered statistically significant.
Results
Patients and Characteristics
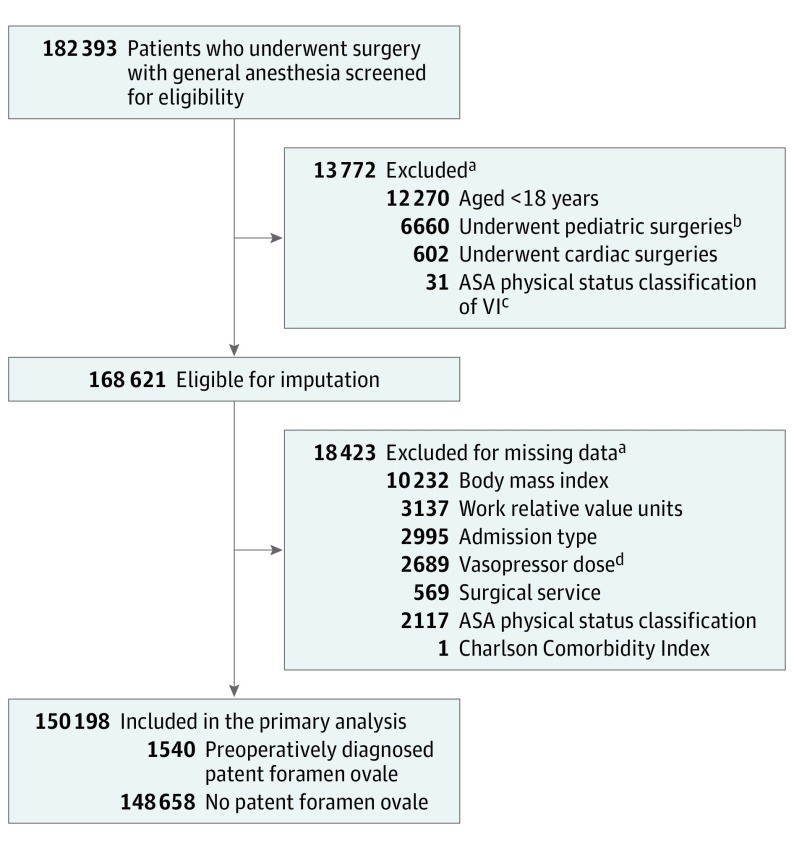
Between January 1, 2007, and December 31, 2015, a total of 182 393 patients were considered for inclusion: 13 772 (7.6%) were excluded due to any of the following exclusion criteria: age younger than 18 years, ASA physical status classification of VI, or having undergone cardiac or pediatric surgeries. Of the remaining 168 621 patients, 18 423 (10.9%) were excluded from the complete case analysis due to missing values in any of the variables used in the regression model (Figure 1). Table 1 shows the baseline and intraoperative characteristics of the study population. Of the 150 198 patients who underwent analysis, 1540 (1.0%) had a preoperatively diagnosed PFO based on ICD-9 and ICD-10 codes. Patients with PFO were older; had a lower body mass index (BMI); higher ASA physical classification status; a higher Charlson comorbidity index; were more likely to have history of smoking, hypertension, diabetes, dyslipidemia, coronary artery disease, myocardial infarction, congestive heart failure, pulmonary edema, pulmonary hypertension, cardiomyopathy, congenital heart disease, atrial fibrillation, valvular heart disease, COPD, transient ischemic attack, ischemic stroke, migraine, chronic kidney disease, hypercoagulable state, deep vein thrombosis, pulmonary embolism, and systemic embolic phenomenon; had more prescriptions of β-blockers, statins, antiplatelet agents, and anticoagulants; underwent more emergency procedures, high-risk procedures, inpatient surgeries, and longer surgeries; had more intraoperative hypotensive minutes; and received higher intraoperative doses of vasopressors, less intraoperative fluids, and more packed red blood cell transfusions. There was no significant difference in sex or procedural complexity measured by work relative value units.
Figure 1. Selection of Patients With vs Without Preoperatively Diagnosed Patent Foramen Ovale and Perioperative Ischemic Stroke Risk.
aSome patients had more than 1 reason for exclusion.
bPediatric surgeries include procedures performed by the pediatric surgical service, which are not limited to patients under 18 years. These procedures may be planned for congenital conditions, for example, fundoplication and repair of pectus excavatum.
cThe American Society of Anesthesiologists (ASA) physical status classification system was used to evaluate patients’ physical state before undergoing anesthesia or surgery. Current definitions include 6 categories (ASA I [normal healthy patient] to ASA VI [patient with brain death]).
dVasopressor dose is the total dose of inotropes and vasopressors used intraoperatively, including epinephrine, norepinephrine, phenylephrine, and dopamine, expressed in milligrams of norepinephrine equivalent.
Table 1. Baseline and Intraoperative Characteristics of Patients With and Without Patent Foramen Ovale.
| Characteristics | No. (%) | P Value | ||
|---|---|---|---|---|
| Total Study Population (N = 150 198) | PFO (n = 1540) | No PFO (n = 148 658) | ||
| Baseline Characteristics and Comorbid Conditions | ||||
| Age, mean (SD), y | 55 (16) | 59 (16) | 55 (16) | <.001 |
| Women | 82 029 (54.6) | 851 (55.3) | 81 178 (54.6) | .61 |
| Body mass index, mean (SD)a | 28.5 (7.1) | 27.9 (7.0) | 28.5 (7.1) | .002 |
| ASA physical status classification, median (IQR)b | 2 (2-3) | 3 (2-3) | 2 (2-3) | <.001 |
| Charlson Comorbidity Index, median (IQR)c | 2 (0-3) | 4 (2-8) | 2 (0-3) | <.001 |
| Hypertension | 63 955 (42.6) | 985 (64.0) | 62 970 (42.4) | <.001 |
| Dyslipidemia | 48 758 (32.5) | 731 (47.5) | 48 027 (32.3) | <.001 |
| Smoking | 30 141 (20.1) | 398 (25.8) | 29 743 (20.0) | <.001 |
| Diabetes | 21 258 (14.2) | 373 (24.2) | 20 885 (14.0) | <.001 |
| Coronary artery disease | 19 654 (13.1) | 547 (35.5) | 19 107 (12.9) | <.001 |
| Valvular heart disease | 15 808 (10.5) | 742 (48.2) | 15 066 (10.1) | <.001 |
| Hypercoagulable state | 14 167 (9.4) | 406 (26.4) | 13 761 (9.3) | <.001 |
| Atrial fibrillation | 12 234 (8.1) | 415 (26.9) | 11 819 (8.0) | <.001 |
| Chronic obstructive pulmonary disease | 11 421 (7.6) | 269 (17.5) | 11 152 (7.5) | <.001 |
| Chronic kidney disease | 11 293 (7.5) | 349 (22.7) | 10 944 (7.4) | <.001 |
| Pulmonary edema | 10 299 (6.9) | 369 (24.0) | 9930 (6.7) | <.001 |
| Congestive heart failure | 9617 (6.4) | 436 (28.3) | 9181 (6.2) | <.001 |
| Systemic embolic phenomenon | 8337 (5.6) | 184 (11.9) | 8153 (5.5) | <.001 |
| Migraine | 6050 (4.0) | 90 (5.8) | 5960 (4.0) | <.001 |
| Cardiomyopathy | 5043 (3.4) | 230 (14.9) | 4813 (3.2) | <.001 |
| Deep vein thrombosis | 3901 (2.6) | 142 (9.2) | 3759 (2.5) | <.001 |
| Ischemic stroke | 3862 (2.6) | 277 (18.0) | 3585 (2.4) | <.001 |
| Myocardial infarction | 2702 (1.8) | 128 (8.3) | 2574 (1.7) | <.001 |
| Pulmonary embolism | 2454 (1.6) | 95 (6.2) | 2359 (1.6) | <.001 |
| Pulmonary hypertension | 2212 (1.5) | 157 (10.2) | 2055 (1.4) | <.001 |
| Transient ischemic attack | 1739 (1.2) | 102 (6.6) | 1637 (1.1) | <.001 |
| Congenital heart disease | 1079 (0.7) | 75 (4.9) | 1004 (0.7) | <.001 |
| Eisenmenger syndrome | 188 (0.1) | 20 (1.3) | 168 (0.1) | <.001 |
| Prescribed medication <28 d before surgery | ||||
| Anticoagulants | 46 563 (31.0) | 771 (50.1) | 45 792 (30.8) | <.001 |
| Statins | 35 437 (23.6) | 608 (39.5) | 34 829 (23.4) | <.001 |
| β-Blockers | 20 423 (13.6) | 482 (31.3) | 19 941 (13.4) | <.001 |
| Antiplatelet drugs | 17 879 (11.9) | 448 (29.1) | 17 431 (11.7) | <.001 |
| Intraoperative Characteristics | ||||
| High-risk procedured | 59 788 (39.8) | 718 (46.6) | 59 070 (39.7) | <.001 |
| Inpatient procedure | 11 1949 (74.5) | 1374 (89.2) | 110 575 (74.4) | <.001 |
| Emergency procedure | 5993 (4.0) | 84 (5.5) | 5909 (4.0) | .003 |
| Packed red blood cell units transfused intraoperatively | 5193 (3.5) | 106 (6.9) | 5087 (3.4) | <.001 |
| Work relative value units, median (IQR)e | 14.5 (8.1-22.0) | 14.7 (8.1-21.8) | 14.5 (8.1-22.0) | .77 |
| Duration of procedure, median (IQR), min | 144 (92-227) | 155 (98-249) | 144 (92-225) | <.001 |
| Intraoperative hypotensive minutes mean arterial pressure <55 mm Hg, median (IQR) | 0 (0-2) | 0 (0-2) | 0 (0-2) | <.001 |
| Total intraoperative norepinephrine equivalent dose, median (IQR), mg | 0.0 (0.0-0.2) | 0.1 (0.0-0.4) | 0.0 (0.0-0.2) | <.001 |
| Total intraoperative fluids, median (IQR), mL | 1250 (800-2000) | 1006 (675-2000) | 1250 (800-2000) | <.001 |
Abbreviations: ASA, American Society of Anesthesiologists; IQR, interquartile range.
Calculated as weight in kilograms divided by height in meters squared.
Evaluates physical state before undergoing anesthesia or surgery (6 categories: ASA I, normal healthy patient to ASA VI, patient with brain death).
Estimates the risk of death by scoring 22 comorbid diseases (each is assigned a score of 1, 2, 3, or 6; total score is used to predict mortality).
Includes surgery for burns, general surgery, neurosurgery, thoracic surgery, transplant surgery, and vascular surgery.
A measure of the value of the services provided by physicians and a marker of the procedural complexity.
Patients with PFO were more likely to have underlying major cardiovascular or thromboembolic conditions at the time of PFO diagnosis (eTable 2 in Supplement 2). Because these coexisting conditions could have biased such patients to the diagnosis of PFO through referral for a dedicated echocardiography study, all of these significant conditions were included in the confounder model for the primary analysis. eTable 3 in Supplement 2 shows details of the type of surgery for patients with and without PFO. Patients with PFO underwent higher frequency counts of anesthesiology and radiology procedures, neurosurgeries, transplant surgeries, and vascular surgeries compared with patients without PFO.
Evaluation of Primary Regression Model
The assumptions underlying the multivariable logistic regression model and the model fit were evaluated. The area under the receiver operating characteristic curve was 0.85 (eFigure 1a in Supplement 2). Assessment of model calibration (eFigure 1b in Supplement 2) and resolution (insert in eFigure 1a in Supplement 2) confirmed that the primary regression model was well-calibrated and had good resolution.
Primary Outcome
A total of 850 (0.6%) perioperative ischemic strokes occurred with 49 (3.2%) among patients with PFO and 801 (0.5%) among patients without PFO (absolute risk difference [RD], 2.6% [95% CI, 1.8%-3.5%]). In adjusted analysis, patients with PFO had an increased risk of perioperative ischemic stroke in the 30 days after surgery compared with patients without PFO (OR, 2.66 [95% CI, 1.96-3.63]; P < .001). This translated to an estimated risk of 5.9 (95% CI, 4.0-7.9) ischemic strokes for every 1000 patients with PFO, an estimated risk of 2.2 (95% CI, 1.9-2.5) for every 1000 surgical patients without PFO, and an adjusted adjusted RD of 0.4% (95% CI, 0.2%-0.6%).
Patients with PFO had an increased risk of large-vessel territory ischemia (37 [2.4%]) compared with patients without PFO (534 [0.4%]) (relative RR, 3.14 [95% CI, 2.21-4.48]; P < .001). Further subtyped according to the Oxfordshire Community Stroke Project classification, the risk of perioperative total anterior stroke was increased among patients with PFO (18 [1.2%]) compared with patients without PFO (214 [0.1%]) (relative RR, 3.66 [95% CI, 2.20-6.08]; P < .001), and risk of posterior circulation stroke was increased among patients with PFO (13 [0.8%]) compared with patients without PFO (193 [0.1%]) (relative RR, 3.14 [95% CI, 1.75-5.63]; P < .001). The risk was not significantly different for partial anterior stroke (6 [0.4%] among patients with PFO vs 127 [0.1%] among patients without PFO; relative RR, 2.28 [95% CI, 0.98-5.30]; P = .06), or for lacunar stroke (0 among patients with PFO vs 37 [<0.1%] among patients without PFO [relative RR, 0.00]; P = .99) (absolute RDs and adjusted absolute RDs are presented in Table 2).
Table 2. Primary, Secondary, and Exploratory Outcomes Among Patients With and Without Patent Foramen Ovale.
| Outcomes | PFO (n = 1540) | No PFO (n = 148 658) | Absolute RD, % (95% CI) | Adjusted Absolute RD, % (95% CI) | OR (95% CI)b | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | Estimated Probability, % (95% CI)a | No. (%) | Estimated Probability, % (95% CI)a | Unadjusted | P Value | Adjustedc | P Value | |||
| Primary | ||||||||||
| Perioperative ischemic stroke | 49 (3.2) | 0.6 (0.4 to 0.8) | 801 (0.5) | 0.2 (0.2 to 0.3) | 2.6 (1.8 to 3.5) | 0.4 (0.2 to 0.6) | 6.07 (4.52 to 8.13) | <.001 | 2.66 (1.96 to 3.63) | <.001 |
| Stroke subtyped | ||||||||||
| Total anterior | 18 (1.2) | 0.2 (0.08 to 0.3) | 214 (0.1) | 0.05 (0.04 to 0.07) | 1.0 (0.5 to 1.6) | 0.1 (0.04 to 0.2) | 8.34 (5.14 to 13.53)e | <.001 | 3.66 (2.20 to 6.08)e | <.001 |
| Partial anterior | 6 (0.4) | 0.07 (0.01 to 0.1) | 127 (0.1) | 0.03 (0.02 to 0.04) | 0.3 (−0.01 to 0.6) | 0.04 (−0.0002 to 0.001) | 4.69 (2.06 to 10.64)e | <.001 | 2.28 (0.98 to 5.30)e | .06 |
| Posterior circulation | 13 (0.8) | 0.2 (0.1 to 0.3) | 193 (0.1) | 0.05 (0.04 to 0.07) | 0.7 (0.3 to 1.2) | 0.1 (0.01 to 0.2) | 6.68 (3.80 to 11.74)e | <.001 | 3.14 (1.75 to 5.63)e | <.001 |
| Lacunar | 0 | 0 (0 to 0) | 37 (<0.1) | 0.002 (−0.002 to 0.006) | −0.02 (−0.03 to −0.02) | −0.002 (−0.006 to 0.002) | 0.00e | .99 | 0.00e | .99 |
| Unclassifiable | 12 (0.8) | 0.1 (0.04 to 0.2) | 230 (0.2) | 0.06 (0.04 to 0.07) | 0.6 (0.2 to 1.1) | 0.0006 (−0.0001 to 0.001) | 5.17 (2.89 to 9.27)e | <.001 | 1.98 (1.09 to 3.61)e | .026 |
| Large-vessel territory strokef | 37 (2.4) | 0.5 (0.3 to 0.6) | 534 (0.4) | 0.1 (0.1 to 0.2) | 2.0 (−14.5 to 18.6) | 0.003 (0.001 to 0.005) | 6.87 (4.91 to 9.63)e | <.001 | 3.14 (2.21 to 4.48)e | <.001 |
| Secondary Outcomes | ||||||||||
| 30-d readmission | 245 (15.9) | 7.0 (6.0 to 7.9) | 11 352 (7.6) | 6.1 (6.0 to 6.3) | 8.3 (6.4 to 10.10) | 0.8 (−0.1 to 1.8) | 2.29 (1.99 to 2.63) | <.001 | 1.15 (0.99 to 1.33) | .07 |
| 30-d mortality | 27 (1.8) | 0.1 (0.06 to 0.2) | 917 (0.6) | 0.1 (0.09 to 0.1) | 1.1 (0.5 to 1.8) | 0.01 (−0.05 to 0.04) | 2.88 (1.95 to 4.23) | <.001 | 0.95 (0.63 to 1.43) | .80 |
| Systemic embolic complications | ||||||||||
| Composite outcomeg | 50 (3.3) | 3.1 (2.3 to 4.0) | 2127 (1.4) | 1.4 (1.3 to 1.4) | 1.8 (0.9 to 2.7) | 1.8 (0.8 to 2.6) | 2.31 (1.74 to 3.07) | <.001 | 2.31 (1.74 to 3.08)h | <.001 |
| Renal artery embolism | 4 (0.3) | 0.3 (0.0 to 0.5) | 74 (0.1) | 0.05 (0.04 to 0.06) | 0.2 (−0.04 to 0.5) | 0.2 (0.04 to 0.2) | 5.23 (1.91 to 14.32) | .001 | 5.15 (1.88 to 14.13)h | .001 |
| Acute limb ischemia | 14 (0.9) | 0.8 (0.5 to 1.3) | 555 (0.4) | 0.4 (0.3 to 0.4) | 0.5 (0.1 to 1.0) | 0.5 (0.03 to 0.9) | 2.44 (1.44 to 4.17) | .001 | 2.31 (1.35 to 3.94)h | .002 |
| Acute intestinal vascular insufficiency | 7 (0.5) | 0.3 (0.08 to 0.6) | 284 (0.2) | 0.1 (0.1 to 0.2) | 0.3 (−0.07 to 0.6) | 0.2 (−0.07 to 0.4) | 2.39 (1.13 to 5.06) | .023 | 2.04 (0.96 to 4.33)h | .064 |
| Acute myocardial infarction | 45 (2.9) | 1.5 (1.1 to 2.0) | 922 (0.6) | 0.4 (0.4 to 0.4) | 2.3 (1.5 to 3.1) | 1.1 (0.7 to 1.6) | 4.82 (3.56 to 6.53) | <.001 | 1.60 (1.13 to 2.27) | .008 |
| Falsification testing | ||||||||||
| Septic shock | 15 (1.0) | 0.09 (0.04 to 0.1) | 525 (0.4) | 0.1 (0.08 to 0.1) | 0.6 (0.1 to 1.1) | −0.01 (−0.06 to 0.04) | 2.78 (1.66 to 4.65) | <.001 | 0.89 (0.52 to 1.52) | .67 |
| Wound complication | 103 (6.7) | 3.0 (2.4 to 3.6) | 6807 (4.6) | 3.3 (3.2 to 3.4) | 2.1 (0.9 to 3.4) | −0.3 (−0.9 to 0.3) | 1.49 (1.22 to 1.83) | <.001 | 0.90 (0.73 to 1.11) | .35 |
| Peptic ulcer disease | 8 (0.5) | 0.3 (0.1 to 0.5)i | 492 (0.3) | 0.2 (0.2 to 0.3)i | 0.2 (−0.2 to 0.5) | 0.06 (−0.15 to 0.27) | 1.57 (0.78 to 3.17) | .21 | 1.23 (0.61 to 2.49)i | .56 |
Abbreviations: IQR, interquartile range; OR, odds ratio; RD, risk difference.
Estimated probabilities were calculated by holding all other covariates at means.
ORs were derived from logistic regression.
If not stated otherwise, adjusted analyses were adjusted for baseline patient characteristics including age, sex, body mass index, American Society of Anesthesiologists physical status classification, and Charlson Comorbidity Index; coexisting conditions such as history of cigarette smoking, hypertension, diabetes, dyslipidemia, coronary artery disease, myocardial infarction, congestive heart failure, pulmonary edema, pulmonary hypertension, cardiomyopathy, congenital heart disease, atrial fibrillation, valvular heart disease, chronic obstructive pulmonary disease, migraine, chronic kidney disease, hypercoagulable state, deep vein thrombosis, pulmonary embolism, and systemic embolic phenomenon; prescription use within 28 days before surgery of β-blockers, statins, antiplatelet agents, and anticoagulants; factors relating to surgery including emergency surgery status, inpatient surgery, high-risk surgical service, duration of surgery, intraoperative hypotensive minutes, intraoperative dose of vasopressors, intraoperative fluid volumes, requirement for packed red blood cells transfusion, and work relative value units.
Stroke subtype by Oxford Community Stroke Project classification.
Relative risk ratio, derived from multinomial logistic regression.
Includes total anterior, partial anterior, and posterior circulation strokes.
Indicates composite outcome of acute embolic events in the extremities, kidneys, spleen, splanchnic circulation, and retina.
Values were adjusted for age, sex, and body mass index.
Values were adjusted for age, sex, body mass index, and the 3 most significant predictors of perioperative stroke (based on the β-coefficients in the primary logistic regression model)—emergency surgery status, inpatient surgery, and high-risk surgical service.
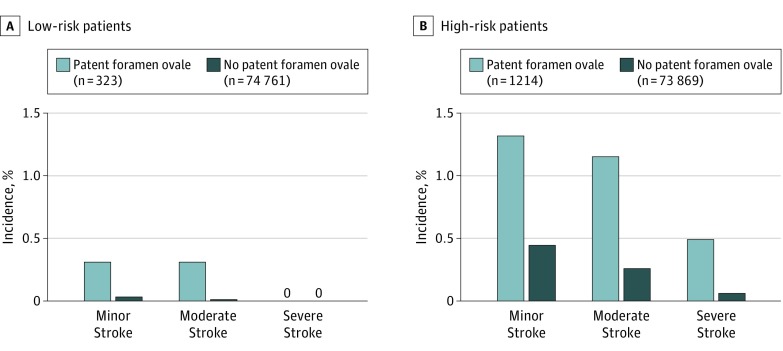
Among patients with stroke, those with PFO had more severe stroke-related neurologic deficit measured by NIHSS (median, 4 [interquartile range {IQR}, 2-10]) than those without PFO (median, 3 [IQR, 1-6]; P = .02). Figure 2 shows the incidence of mild, moderate, and severe strokes (as defined by NIHSS) in patients with and without PFO.
Figure 2. Incidence of Stroke Among Low-Risk and High-Risk Stroke Patients, Stratified by Clinical Severity.
The baseline patent foramen ovale (PFO)-independent risk of stroke was determined by a priori selected–variables (section 6.6 in Supplement 2). The study population was divided into low stroke risk and high stroke risk based on this baseline risk (sample size differs because 31 patients with American Society of Anesthesiologists [ASA] physical status class V were excluded due to failure to calculate the probability score [section 6.6 and eTable 5 in Supplement 2]). Stroke severity was stratified into minor stroke (National Institutes of Health Stroke Scale [NIHSS] scores 1-4), moderate stroke (NIHSS 5-14), and severe stroke (NIHSS 15-42). Patients with PFO had a higher incidence of stroke across all subgroups. For low-risk patients, the incidences of minor stroke (1 of 323 [0.31%] vs 24 of 74 761 [0.03%]; P = .006) and moderate stroke (1 of 323 [0.31%] vs 8 of 74 761 [0.01%]; P < .001) were higher among patients with PFO; there were no severe strokes. For high-risk patients, the incidences of minor stroke (16 of 1214 [1.32%] vs 331 of 73 869 [0.45%]; P < .001), moderate stroke (14 of 1214 [1.15%] vs 194 of 73 869 [0.26%]; P < .001), and severe stroke (6 of 1214 [0.49%] vs 49 of 73 869 [0.07%]; P < .001) were higher among patients with PFO.
Sensitivity Analyses
Of the 1540 patients with PFO, 1521 (98.8%) were successfully matched at a 1:5 ratio (Table 3). The propensity score–matched cohort confirmed the association between PFO and stroke (49 of 1538 [3.2%] patients with PFO vs 77 of 7656 [1.0%] patients without PFO; OR, 3.16 [95% CI, 2.19-4.51]; P < .001; section 6.4 in Supplement 2).
Table 3. Baseline and Intraoperative Characteristics of Patients With and Without Patent Foramen Ovale in a 1:5 Propensity-Matched Cohort.
| Characteristics | No. (%) | Standardized Difference | |
|---|---|---|---|
| PFO (n = 1538) | No PFO (n = 7656) | ||
| Baseline Characteristics and Comorbid Conditions | |||
| Age, mean (SD), y | 59 (16) | 59 (17) | 0.019 |
| Women | 849 (55.2) | 4219 (55.1) | 0.008 |
| Body mass index, mean (SD)a | 28.0 (7.0) | 28.0 (7.1) | 0.009 |
| ASA physical status classification, median (IQR)b | 3 (2-3) | 3 (2-3) | 0.033 |
| Charlson Comorbidity Index, median (IQR)c | 4 (2-8) | 3 (2-8) | 0.028 |
| Hypertension | 983 (63.9) | 4868 (63.6) | 0.023 |
| Valvular heart disease | 740 (48.1) | 1999 (26.1) | 0.479 |
| Dyslipidemia | 731 (47.5) | 3593 (46.9) | 0.024 |
| Coronary artery disease | 545 (35.4) | 2307 (30.1) | 0.140 |
| Congestive heart failure | 434 (28.2) | 1979 (25.8) | 0.054 |
| Atrial fibrillation | 413 (26.9) | 1950 (25.5) | 0.044 |
| Hypercoagulable state | 404 (26.3) | 1244 (16.2) | 0.261 |
| Smoking | 398 (25.9) | 2015 (26.3) | 0.006 |
| Diabetes | 372 (24.2) | 1828 (23.9) | 0.009 |
| Pulmonary edema | 367 (23.9) | 1499 (19.6) | 0.082 |
| Chronic kidney disease | 347 (22.6) | 1602 (20.9) | 0.034 |
| Ischemic stroke | 276 (179) | 456 (6.0) | 0.372 |
| Chronic obstructive pulmonary disease | 268 (17.4) | 1275 (16.7) | 0.014 |
| Cardiomyopathy | 229 (14.9) | 856 (11.2) | 0.106 |
| Systemic embolic phenomenon | 184 (12.0) | 778 (10.2) | 0.059 |
| Pulmonary hypertension | 156 (10.1) | 418 (5.5) | 0.179 |
| Deep vein thrombosis | 140 (9.1) | 451 (5.9) | 0.118 |
| Myocardial infarction | 126 (8.2) | 537 (7.0) | 0.046 |
| Transient ischemic attack | 102 (6.6) | 203 (2.7) | 0.208 |
| Pulmonary embolism | 94 (6.1) | 273 (3.6) | 0.108 |
| Migraine | 90 (5.9) | 297 (3.9) | 0.060 |
| Congenital heart disease | 75 (4.9) | 119 (1.6) | 0.199 |
| Eisenmenger syndrome | 20 (1.3) | 16 (0.2) | 0.114 |
| Prescribed medication <28 d before surgery | |||
| Anticoagulants | 769 (50.0) | 3802 (49.7) | 0.007 |
| Statins | 607 (39.5) | 2978 (38.9) | 0.007 |
| β-Blockers | 481 (31.3) | 2354 (30.7) | 0.030 |
| Antiplatelet drugs | 446 (29.0) | 2149 (28.1) | 0.027 |
| Intraoperative Characteristics | |||
| Inpatient procedure | 1372 (89.2) | 6817 (89.0) | 0.006 |
| Packed red blood cell units transfused intraoperatively | 106 (6.9) | 291 (6.4) | 0.017 |
| Emergency procedure | 84 (5.5) | 378 (4.9) | 0.000 |
| High-risk procedured | 17 (46.6) | 3550 (46.4) | 0.008 |
| Work relative value units, median (IQR)e | 14.7 (8.1-21.8) | 15.0 (8.2-22.4) | 0.017 |
| Duration of procedure, median (IQR), min | 155 (98-249) | 150 (94-236) | 0.090 |
| Intraoperative hypotensive minutes mean arterial pressure <55 mm Hg, median (IQR) | 0 (0-2) | 0 (0-2) | 0.033 |
| Total intraoperative norepinephrine equivalent dose, median (IQR), mg | 0.1 (0.0-0.4) | 0.1 (0.0-0.4) | 0.028 |
| Total intraoperative fluids, median (IQR), mL | 1007 (700-2000) | 1200 (750-2000) | 0.009 |
Abbreviations: ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; MAP, mean arterial pressure; PFO, patent foramen ovale.
Calculated as weight in kilograms divided by height in meters squared.
Evaluates physical state before undergoing anesthesia or surgery (6 categories: ASA I, normal healthy patient to ASA VI, patient with brain death).
Estimates the risk of death by scoring 22 comorbid diseases (each is assigned a score of 1, 2, 3, or 6; total score is used to predict mortality).
Includes surgery for burns, general surgery, neurosurgery, thoracic surgery, transplant surgery, and vascular surgery.
A measure of the value of the services provided by physicians and a marker of the procedural complexity.
There were 29 629 (19.7%) patients in the cohort with history of an echocardiogram performed in the same health care system (eTable 6 in Supplement 2). Subgroup analyses of the association between PFO and stroke in this cohort confirmed the study findings (32 of 1162 [2.8%] patients with PFO vs 335 of 28 467 [1.2%] patients without PFO; OR, 1.86 [95% CI, 1.28-2.72]; P = .001; [section 6.5 of Supplement 2]). Analyses further stratified by patients with TEE prior to surgery (4094 [904 with PFO {22.1%}]; [section 7.9.1 subsection 1 of Supplement 2]) and patients with contrast echocardiography (echocardiography with agitated saline injection) prior to surgery (4043 [1139 with PFO {28.2%}]; section 7.9.1 subsection 2 of Supplement 2) reiterated the association between PFO and stroke (37 of 904 [4.1%] patients with PFO vs 44 of 3190 [1.4%] patients without PFO; adjusted OR, 2.06 [95% CI, 1.15-3.68]; P = .02) in patients with TEE; and (45 of 1139 [4.0%] patients with PFO vs 67of 2904 [2.3%] patients without PFO; adjusted OR, 1.86 [95% CI, 1.26-2.74]; P = .002 in patients with contrast echocardiography; [section 7.9.1 of Supplement 2 for further details]).
The area under the curve for the probability score of baseline PFO-independent risk of perioperative ischemic stroke was 0.84 (section 6.6 in Supplement 2). The study population was subdivided by median split into 2 equally sized groups (low stroke risk and high stroke risk) based on this baseline stroke risk (eTable 5 in Supplement 2). For individuals at low risk of stroke, the estimated probability of stroke with PFO was 1.4% (95% CI, 0.2%-2.9%) vs 0.1% (95% CI, 0.1%-0.2%) without PFO; (adjusted OR, 15.92 [95% CI, 4.92-51.53]; P < .001). For individuals at high risk of stroke, the estimated probability of stroke with PFO was 0.8% (95% CI, 0.5%-1.0%) vs 0.3% (95% CI, 0.3%-0.4%) without PFO; (adjusted OR, 3.80 [95% CI, 2.81-5.15]; P < .001). The RR estimate of PFO on perioperative ischemic stroke was modified by patients’ PFO-independent baseline risk of stroke (interaction term, PFO × low stroke risk; OR, 4.59 [95% CI, 1.36-15.5]; P for interaction = .014). Specifically, the PFO-attributable risk of stroke in patients at low baseline stroke risk was higher (adjusted absolute RD, 1.3% [95% CI, −0.3% to 2.8%]) compared with patients at high baseline stroke risk (adjusted absolute RD, 0.5% [95% CI, 0.2%-0.7%) (section 6.6 in Supplement 2).
In the primary analysis, the comorbidities in multivariable model did not include history of transient ischemic attack or ischemic stroke since they may have also been associated with a PFO. As a sensitivity test, including a history of transient ischemic attack (OR, 2.32 [95% CI, 1.70-3.18]; P < .001) or history of ischemic stroke (OR, 1.55 [95% CI, 1.13-2.14]; P = .007) in the confounder model did not change the primary study results.
The incidence of the ICD-based outcome of perioperative ischemic stroke was 2155 (1.4%) (136 [8.8%] in the PFO group and 2019 [1.4%] in the group without PFO). In adjusted analysis, the odds of experiencing a stroke in the PFO group was greater (OR, 2.91 [95% CI, 2.38-3.56]; P < .001) (section 6.1 in Supplement 2).
Falsification testing showed that the following perioperative outcomes were not significantly associated with having a diagnosis of PFO: septic shock (15 [1.0%] with PFO vs 525 [0.4%] without PFO; OR, 0.89 [95% CI, 0.52-1.52]; P = .67), wound complication (103 [6.7%] with PFO vs 6807 [4.6%] without PFO; OR, 0.90 [95% CI, 0.73-1.11]; P = .35), and peptic ulcer disease (8 [0.5%] with PFO vs 492 [0.3%] without PFO; OR, 1.23 [95% CI, 0.61-2.49]; P = .56) (Table 2; eFigure 2 and section 6.7 in Supplement 2). In further falsification analyses, tricuspid valve disorders were not significantly associated with stroke in the complete case cohort (72 of 4919 [1.5%] with tricuspid valve disorder vs 778 of 145 279 [0.5%] without tricuspid valve disorder; OR, 0.99 [95% CI, 0.75-1.30]; P = .93) or in the cohort with history of an echocardiogram (67 of 4548 [1.5%] with tricuspid valve disorder vs 300 of 25 081 without tricuspid valve disorder [1.2%]; OR, 0.89 [95% CI, 0.66-1.19]; P = .43) (section 7.9.1 subsection 4 in Supplement 2).
A total of 7 variables in the primary regression model had missing data (section 6.9 and eFigure 3 in Supplement 2). Body mass index, the variable which had the largest amount of missing data, had 10 232 (6.1%) missing values. Multiple imputation was conducted, and the imputed cohort included all 18 423 (10.9%) patients who were excluded due to missing values in any of the variables used in the regression model. The model estimate of PFO on risk of stroke from the imputed data set was consistent with the complete case cohort (53 of 1788 [3.0%] with PFO vs 906 of 166 833 [0.5%] without PFO; OR, 2.59 [95% CI, 1.93-3.48]; P < .001). The details of additional sensitivity analyses are provided in Supplement 2.
Secondary Outcomes
In multivariable analyses, having a PFO was not significantly associated with increased rate of 30-day readmission (245 [15.9%] in patients with PFO vs 11 352 [7.6%] in patients without PFO; adjusted OR, 1.15 [95% CI, 0.99-1.33]; P = .07; adjusted absolute RD, 0.8% [95% CI, −0.1% to 1.8%]). There was no significant difference in 30-day mortality between patients with and without PFO (27 [1.8%] in patients with PFO vs 917 [0.6%] in patients without PFO; OR, 0.95 [95% CI, 0.63-1.43]; P = .80; adjusted absolute RD, 0.01%; 95% CI [−0.05% to 0.04%]). Detailed results of secondary outcomes are presented in Table 2.
Exploratory Analyses
The relationship between PFO and other embolic complications was analyzed. PFO was associated with an increased risk of perioperative systemic embolic complications within 30 days of surgery (a composite of acute embolic events in the extremities, kidneys, spleen, splanchnic circulation, and retina within 30 days after surgery) (50 [3.3%] with PFO vs 2127 [1.4%] without PFO; adjusted OR, 2.31 [95% CI, 1.74-3.08]; P < .001). PFO was associated with increased risk of acute limb ischemia (14 [0.9%] with PFO vs 555 [0.4%] without PFO; adjusted OR, 2.31 [95% CI, 1.35-3.94]; P = .002), renal artery embolism (4 [0.3%] with PFO vs 74 [0.1%] without PFO; adjusted OR, 5.15 [95% CI, 1.88-14.13]; P = .001), and acute myocardial infarction (45 [2.9%] with PFO vs 922 [0.6%] without PFO; adjusted OR, 1.60 [95% CI, 1.13-2.27]; P = .008) (Table 2).
Discussion
In this cohort of 150 198 adult patients undergoing noncardiac surgery, a preoperatively diagnosed PFO was associated with an increased risk of perioperative ischemic stroke within 30 days of surgery. The PFO-attributable RR of stroke was more significantly increased among patients who were otherwise at lower risk of stroke compared with those at higher risk of stroke. The association between PFO and stroke remained consistent after adjustment for risk factors of ischemic stroke and multiple sensitivity analyses, including stratified analyses in patients who had received diagnostic tests sensitive for the detection of PFO (TEE or contrast echocardiography). PFO was associated with more large-vessel territory strokes, strokes with more severe neurological deficits, and an increased risk of systemic embolic complications, potentially providing a mechanistic link explaining the actual etiology of the stroke.
PFO is the persistence of an embryonic defect in the atrial septum into adulthood. Right-to-left intracardiac shunting occurs across the PFO when there is reversal of the normal interatrial pressure gradient, leading to the putative mechanism of paradoxical embolism and ischemic stroke. This phenomenon may be exacerbated by different factors in the perioperative period. Intraoperatively, right-to-left shunting may be more likely to occur as a result of hemorrhage, mechanical ventilation, patient positioning, and the fall in systemic vascular resistance due to administration of anesthesia. Postoperatively, the state of systemic inflammation and hypercoagulability in the setting of withdrawal of regular antiplatelet or anticoagulation treatment and complications such as deep vein thrombosis, may interplay to create a high-risk setting for thromboembolism. The markedly higher incidence of ischemic strokes at 3.2% within a narrow time period of 30 days after surgery in the study patients with PFO, in comparison with a reported annual stroke incidence of 1.2% to 1.7% in general populations with PFO, highlights the excess stroke risk in the perioperative period for patients with PFO—at least those in whom it was clinically diagnosed prior to surgery. Despite the pathophysiological plausibility, there are sparse clinical data to address their association. There were only 2 studies that examined the relationship of PFO and perioperative stroke. In 13 092 patients undergoing cardiac surgery, investigators did not find any difference in incidence of postoperative stroke between patients with and without an intraoperative incidental finding of PFO. However, that study excluded all patients with preoperative diagnoses of PFO or atrial septal defects, limiting the generalizability of the findings to addressing the biological effects of PFO on perioperative stroke. A more recent study examined the relationship between PFO and stroke among patients who underwent total hip arthroplasty in the Nationwide Inpatient Sample. Based on a 3:1 propensity-matched cohort including 252 patients with PFO and 756 control participants, the authors showed that the risk of perioperative ischemic stroke was 29 times greater for patients with PFO and atrial septal defects.
In the current study, PFO-related strokes were more frequently large-vessel territory strokes and strokes with more severe neurological deficits. Patients with PFO also experienced an excess of perioperative complications due to systemic embolism, including acute limb ischemia and renal artery embolism. These data provide support for the view of increased vulnerability to thromboembolic complications in patients with PFO during the perioperative period. It is clinically important that patients with PFO in the cohort had more severe strokes with a median NIHSS of 4 compared with 3 among patients without PFO. The correlation of the initial NIHSS following a stroke with outcomes is well-established—each additional point on the NIHSS decreases the likelihood of excellent outcomes at 3 months by 17%.
An important finding from this study was that the PFO-attributable risk of perioperative stroke was highest among patients with an otherwise low probability of perioperative ischemic stroke based on coexisting cardiovascular risk factors and intraoperative characteristics. This is in line with most previous studies in nonperioperative cohorts with cryptogenic stroke, which showed that the significance of PFO in increasing the risk for stroke is higher in patients younger than 55 years with cryptogenic stroke.
There are increasing data to support different strategies of stroke prevention in patients with PFO. The National Institute for Health and Care Excellence–accredited guideline for stroke, and guidelines from the American Heart Association and American Stroke Association recommend antiplatelet therapy for secondary stroke prevention in the absence of another indication for anticoagulation. More recently, several randomized clinical trials demonstrated the value of PFO closure in subgroups of patients with cryptogenic stroke, and existing guidelines for secondary stroke prevention may be modified to reflect these important new findings. However, there are no guidelines for management of cases of patients undergoing surgery. In clinical practice, routine prophylactic antiplatelet or anticoagulation therapy is frequently withheld in the perioperative period. A recent consensus statement on the perioperative care of patients at high risk for stroke did not identify patients with PFO as a high-risk group. The current study identified a potentially actionable subgroup of patients scheduled for a surgical procedure who present to anesthesiologists and surgeons with preoperatively diagnosed PFO. Future studies are required to examine if these patients would benefit from intensifying stroke-preventive measures in the perioperative period (eg, an individualized risk-benefit assessment with regards to timing and choice of perioperative antithrombotic therapy, modified transfusion thresholds, or preoperative PFO closure among select patients).
The strength of this study is the generalizability of the results to a large patient population undergoing noncardiac surgery with general anesthesia. Patients undergoing cardiac surgery were excluded for 2 reasons: these patients represent a group with a potentially different stroke mechanism—one that is more closely related to surgical factors, duration of cardioplegia, and air embolism, rather than venous thrombosis and paradoxical embolism—which was the biological hypothesis being tested; moreover, some but not all cardiac surgeons routinely close PFO encountered during cardiac surgery, leading to potential bias.
Limitations
This study had several limitations. First, the observational nature of the study and the use of administrative data and billing codes confer risks of unmeasured confounding and bias, though the question of any association between PFO and stroke could not be studied with a randomized study. Second, the minimal clinically important difference for the present study was based on a trial of PFO closure for secondary stroke prevention, and therefore may not have been directly applicable to the clinical scenario of postoperative stroke. Third, the cohort included only patients who were extubated at the end of procedure; thus, it is possible that the stroke risk among patients who remain intubated after surgery is different.
Fourth, although only preoperatively diagnosed PFO cases were considered, it is very likely that there was underidentification of PFO in the non-PFO group, which would have biased toward the null. Fifth, the indication for referral for the test that identified PFO could also be a confounding factor representing a risk factor for stroke. However, sensitivity analyses in those patients who had undergone prior to surgery a diagnostic workup sensitive for the detection of PFO, such as TEE or contrast echocardiography, showed a PFO prevalence of 22% to 28%, compatible with most major reports from the general population. The significant association of PFO with an increased risk of perioperative ischemic stroke in subgroups of patients with TEE and contrast TEE further supports the view that the observed association between PFO and perioperative stroke is unlikely to be exclusively explained by a workup bias.
Conclusions
Among adult patients undergoing noncardiac surgery at 3 hospitals, having a preoperatively diagnosed PFO was significantly associated with increased risk of perioperative ischemic stroke within 30 days after surgery. Further research is needed to confirm these findings and to determine whether interventions would decrease this risk.
IRB Protocol
eFigure 1a. Receiver operating characteristic (ROC) curve of the primary regression model
eFigure 1b. Calibration plot of the primary regression model
eFigure 2. Power analysis for falsification tests
eFigure 3. Histogram of variables with missing data and the pattern of missingness
eTable 1. International classification of diseases, ninth and tenth edition (ICD-9/10) diagnostic codes used to define exposure, outcome, and confounder variables
eTable 2. Characterization of major cardiovascular and thromboembolic conditions at the time of PFO diagnosis
eTable 3. Type of surgery
eTable 4. Sample size and power calculation
eTable 5. Baseline and intraoperative characteristics of subgroups stratified by baseline PFO-independent risk of stroke
eTable 6. Baseline and intraoperative characteristics of subgroups stratified by history of echocardiography prior to surgery
Statistical Analysis Plan
Abbreviations List
- NIHSS
National Institutes of Health Stroke Scale
- PFO
patent foramen ovale
- TEE
transesophageal
References
- 1.Weiser TG, Haynes AB, Molina G, et al. . Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet. 2015;385(suppl 2):S11. [DOI] [PubMed] [Google Scholar]
- 2.Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology. 2011;114(6):1289-1296. [DOI] [PubMed] [Google Scholar]
- 3.Selim M. Perioperative stroke. N Engl J Med. 2007;356(7):706-713. [DOI] [PubMed] [Google Scholar]
- 4.Mrkobrada M, Hill MD, Chan MT, et al. . Covert stroke after non-cardiac surgery: a prospective cohort study. Br J Anaesth. 2016;117(2):191-197. [DOI] [PubMed] [Google Scholar]
- 5.Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59(1):17-20. [DOI] [PubMed] [Google Scholar]
- 6.Saver JL, Carroll JD, Thaler DE, et al. ; RESPECT Investigators . Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377(11):1022-1032. [DOI] [PubMed] [Google Scholar]
- 7.Søndergaard L, Kasner SE, Rhodes JF, et al. ; Gore REDUCE Clinical Study Investigators . Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377(11):1033-1042. [DOI] [PubMed] [Google Scholar]
- 8.Mas JL, Derumeaux G, Guillon B, et al. ; CLOSE Investigators . Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 2017;377(11):1011-1021. [DOI] [PubMed] [Google Scholar]
- 9.Lechat P, Mas JL, Lascault G, et al. . Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318(18):1148-1152. [DOI] [PubMed] [Google Scholar]
- 10.Mas JL, Arquizan C, Lamy C, et al. ; Patent Foramen Ovale and Atrial Septal Aneurysm Study Group . Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;345(24):1740-1746. [DOI] [PubMed] [Google Scholar]
- 11.Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology. 2000;55(8):1172-1179. [DOI] [PubMed] [Google Scholar]
- 12.Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: incidental or pathogenic? Stroke. 2009;40(7):2349-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins GJ Jr, Barber JA, Zajtchuk R, Vanek D, Malogne LA. The effects of operative stress on the coagulation profile. Am J Surg. 1977;133(5):612-616. [DOI] [PubMed] [Google Scholar]
- 14.American Society of Anesthesiologists; ASA Physical Status Classification System https://www.asahq.org/resources/clinical-information/asa-physical-status-classification-system. Accessed January 9, 2018.
- 15.Timm FP, Houle TT, Grabitz SD, et al. . Migraine and risk of perioperative ischemic stroke and hospital readmission: hospital based registry study. BMJ. 2017;356:i6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337(8756):1521-1526. [DOI] [PubMed] [Google Scholar]
- 17.Brott T, Adams HP Jr, Olinger CP, et al. . Measurements of acute cerebral infarction: a clinical examination scale. 1989;20(7):864-870. [DOI] [PubMed] [Google Scholar]
- 18.Carroll JD, Saver JL, Thaler DE, et al. . Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368(12):1092-1100. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. [DOI] [PubMed] [Google Scholar]
- 20.Cundiff DK. Clinical evidence for rebound hypercoagulability after discontinuing oral anticoagulants for venous thromboembolism. Medscape J Med. 2008;10(11):258. [PMC free article] [PubMed] [Google Scholar]
- 21.Meissner I, Khandheria BK, Heit JA, et al. . Patent foramen ovale: innocent or guilty? evidence from a prospective population-based study. J Am Coll Cardiol. 2006;47(2):440-445. [DOI] [PubMed] [Google Scholar]
- 22.Di Tullio MR, Sacco RL, Sciacca RR, Jin Z, Homma S. Patent foramen ovale and the risk of ischemic stroke in a multiethnic population. J Am Coll Cardiol. 2007;49(7):797-802. [DOI] [PubMed] [Google Scholar]
- 23.Krasuski RA, Hart SA, Allen D, et al. . Prevalence and repair of intraoperatively diagnosed patent foramen ovale and association with perioperative outcomes and long-term survival. JAMA. 2009;302(3):290-297. [DOI] [PubMed] [Google Scholar]
- 24.Perfetti DC, Chughtai M, Boylan MR, Naziri Q, Maheshwari AV, Mont MA. Atrial septal defect increases the risk for stroke after total hip arthroplasty. J Arthroplasty. 2017;32(10):3152-3156. [DOI] [PubMed] [Google Scholar]
- 25.Adams HP Jr, Davis PH, Leira EC, et al. . Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999;53(1):126-131. [DOI] [PubMed] [Google Scholar]
- 26.Di Tullio M, Sacco RL, Gopal A, Mohr JP, Homma S. Patent foramen ovale as a risk factor for cryptogenic stroke. Ann Intern Med. 1992;117(6):461-465. [DOI] [PubMed] [Google Scholar]
- 27.Royal College of Physicians; Intercollegiate Stroke Working Party National clinical guideline for stroke; 2016. https://www.strokeaudit.org/SupportFiles/Documents/Guidelines/2016-National-Clinical-Guideline-for-Stroke-5t-(1).aspx. Accessed January 9, 2018.
- 28.Kernan WN, Ovbiagele B, Black HR, et al. ; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Council on Peripheral Vascular Disease . Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160-2236. [DOI] [PubMed] [Google Scholar]
- 29.Vaduganathan M, Qamar A, Gupta A, et al. . Patent foramen ovale closure for secondary prevention of cryptogenic stroke: updated meta-analysis of randomized clinical trials. Am J Med. 2017;S0002-9343(17):31219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mashour GA, Moore LE, Lele AV, Robicsek SA, Gelb AW. Perioperative care of patients at high risk for stroke during or after non-cardiac, non-neurologic surgery: consensus statement from the Society for Neuroscience in Anesthesiology and Critical Care*. J Neurosurg Anesthesiol. 2014;26(4):273-285. [DOI] [PubMed] [Google Scholar]
- 31.Meissner I, Whisnant JP, Khandheria BK, et al. . Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study: Stroke Prevention: Assessment of Risk in a Community. Mayo Clin Proc. 1999;74(9):862-869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IRB Protocol
eFigure 1a. Receiver operating characteristic (ROC) curve of the primary regression model
eFigure 1b. Calibration plot of the primary regression model
eFigure 2. Power analysis for falsification tests
eFigure 3. Histogram of variables with missing data and the pattern of missingness
eTable 1. International classification of diseases, ninth and tenth edition (ICD-9/10) diagnostic codes used to define exposure, outcome, and confounder variables
eTable 2. Characterization of major cardiovascular and thromboembolic conditions at the time of PFO diagnosis
eTable 3. Type of surgery
eTable 4. Sample size and power calculation
eTable 5. Baseline and intraoperative characteristics of subgroups stratified by baseline PFO-independent risk of stroke
eTable 6. Baseline and intraoperative characteristics of subgroups stratified by history of echocardiography prior to surgery
Statistical Analysis Plan