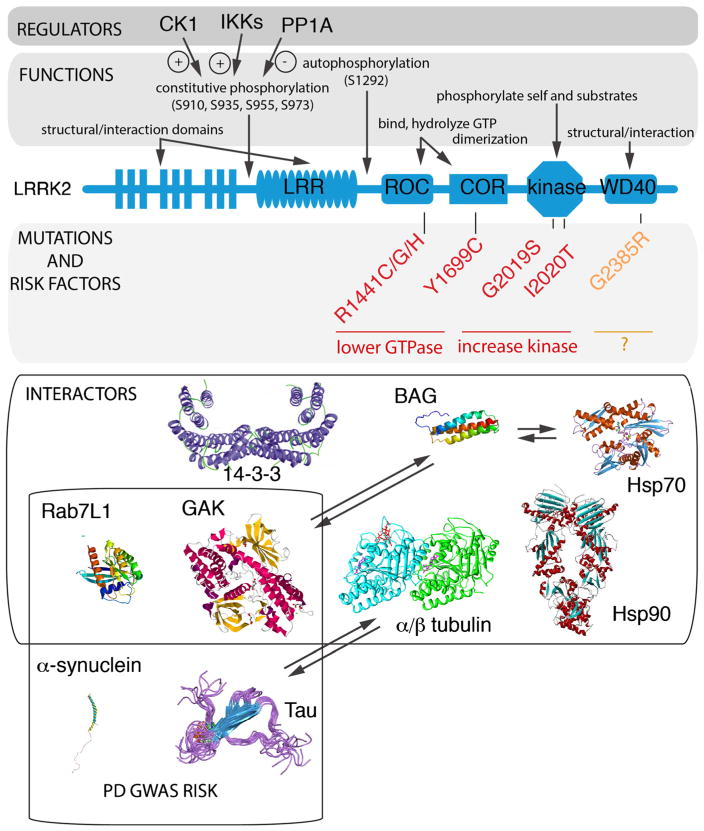
Fig. 1.
LRRK2, mutations, regulation, and interactions. The domain structure of LRRK2 is shown in the center of the figure (blue) and contains (from N- to C-termini) leucine-rich repeats (LRR), a Ras of complex protein (ROC) and C-terminal of ROC (COR) bidomain, a kinase domain, and a WD40 domain. Above the diagram are indications of the biochemical functions of each region and, above those, are some known regulators of phosphorylation status. Below the outline are the major Mendelian mutations (red) and one of the more common risk factors (orange) and an indication of their effects on the measurable biochemical activities of LRRK2. Finally, in the lower portion of the figure, some of the more reliable protein interactions are shown; all of these have been reported in more than one paper and with more than one technique used apart from GAK (cyclin G-associated kinase) which has to date only been reported once. This is included because it is one of two candidate risk factor genes identified by GWAS. Two additional genes that are important for sporadic PD risk are a-synuclein and tau, which have not yet been shown to bind directly to LRRK2 but do appear to be functionally related to some of the pathways in which LRRK2 is involved