Figure 2.5. Tumor-promoting roles of autophagy.
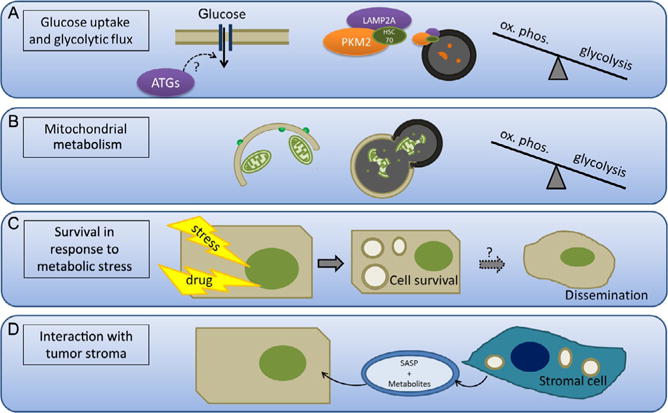
(A) Autophagy promotes glucose uptake and glycolytic flux. Autophagy has been shown to promote glucose uptake in cancer cells, although the mechanism remains to be elucidated. Additionally, increased chaperone-mediated autophagy (CMA) promotes degradation of PKM2, a rate-limiting glycolytic enzyme. Thus, CMA can control the rate of flux through glycolysis and whether glycolytic intermediates are used for energy production or anaplerosis. (B) Autophagy selectively degrades the mitochondria and therefore the machinery necessary for fatty acid oxidation and oxidative phosphorylation. This enhances the shift to glycolysis, which is characteristic of cancer cells. (C) Autophagy promotes survival in response to metabolic stress such as growth factor deprivation, acidic environment, and ER stress by recycling cytoplasmic material in order to maintain the basal energy state and clear damaged, misfolded proteins. This process may also be important for survival during tumor dissemination and metastasis. (D) Autophagy in stromal cells induced by the hypoxic and acidic tumor microenvironment promotes the secretion of metabolites and growth signals via senescence-associated secretion phenotype (SASP) that enhances tumor cell growth.
