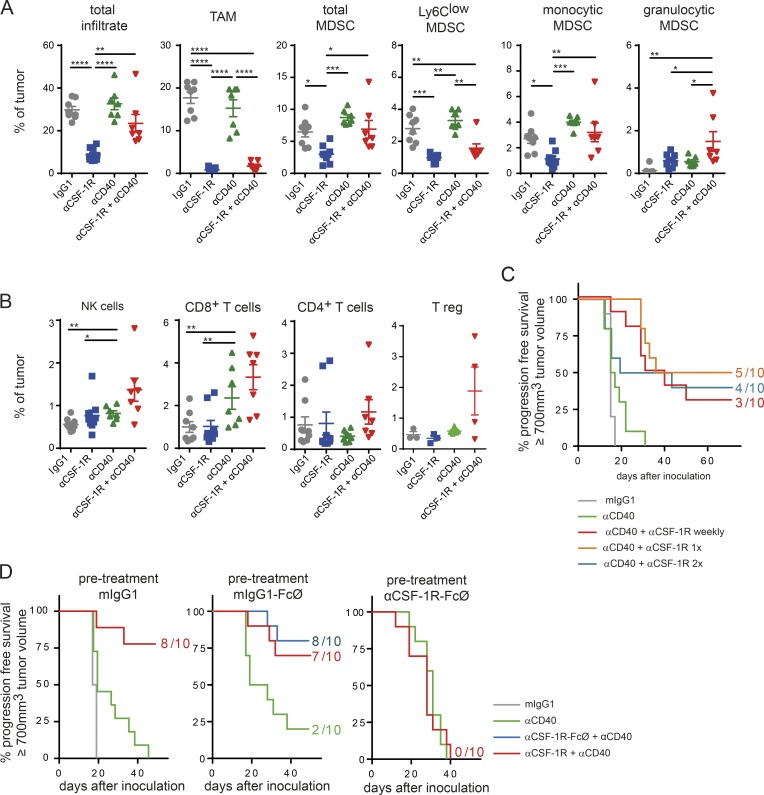
Figure 2.
TAMs are required for αCSF-1R+αCD40-mediated tumor rejection. (A and B) Immune infiltrate of MC38 tumors on day 10 upon start of treatment. MC38 tumor–bearing mice were treated with either 30 mg/kg mIgG1, 30 mg/kg αCSF-1R weekly, 4 mg/kg αCD40 once, or a combination of both targeting antibodies. Data shown in A and B are pooled from two independent experiments, and T reg cells were assessed in the third experiment. Graphs show means ± SEM; statistical analysis by one-way ANOVA and Tukey correction (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). (C) A single combined dose of αCSF-1R and αCD40 antibodies is sufficient for tumor rejection. MC38 tumor–bearing mice were treated using 30 mg/kg mIgG1, 4 mg/kg αCD40 once, or in combination with 30 mg/kg αCSF-1R given weekly (four times total), once (first treatment day), or twice on first and second treatment day. This experiment is exemplary out of two independent experiments. (D) Predepletion of macrophages completely abolishes tumor rejection in MC38 tumor–bearing mice. Mice were inoculated with MC38 tumors and received on days 3 and 9 upon inoculation of either 30 mg/kg mIgG1, mIgG1 incompetent in binding to FcγR (mIgG1-FcØ), or αCSF-1R clone 2G2 incompetent in binding to FcγR (αCSF-1R–FcØ). On day 11, at a mean tumor volume of 120 mm3, mice were treated with a single dose of either 130 mg/kg mIgG, 4 mg/kg αCD40, or a combination of 30 mg/kg αCSF-1R+αCD40 (FcγR competent or incompetent versions). Depletion of TAMs was confirmed by flow cytometry on day 11 from scout animals (Fig. S3 D). (C and D) Mice were graphically censored once the tumor size reached ≥700 mm3, and numbers in graphs indicate the amount of tumor-free mice within the specific group from n = 10 for all groups depicted.