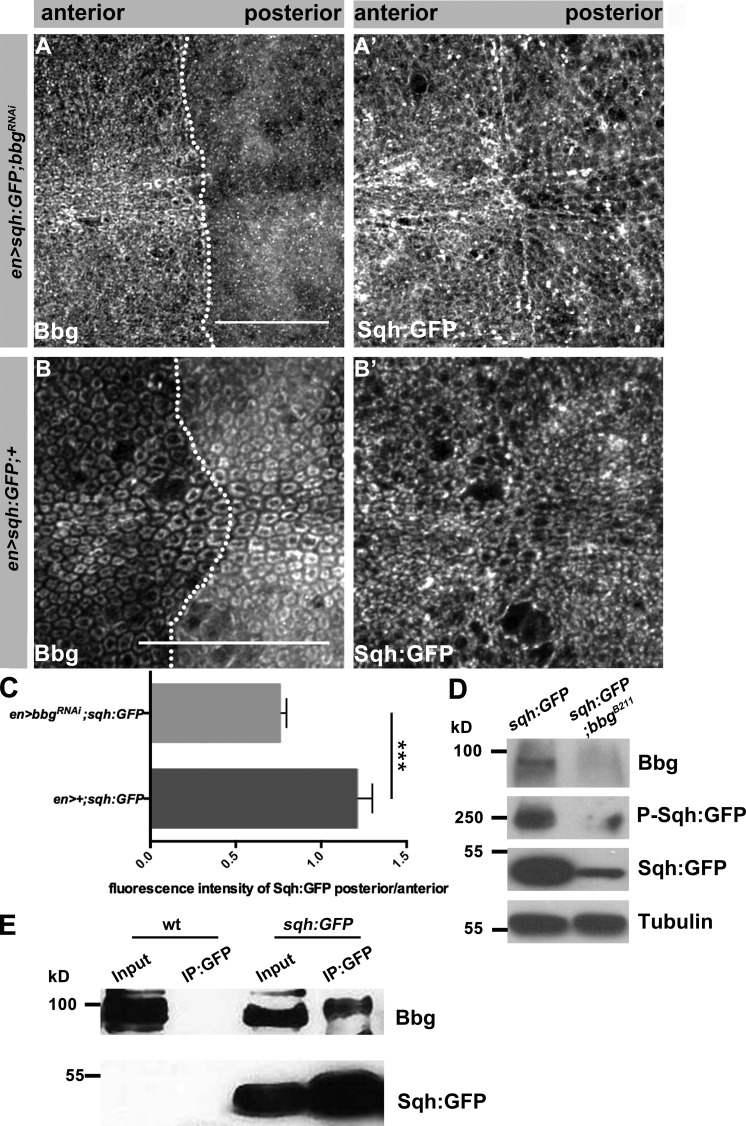
Figure 6.
Bbg is in the same protein complex as Sqh and stabilizes Sqh in the apical cytocortex. (A and A′) Pouch of en-Gal4, UAS:RFP, sqh-GFP; UAS-bbgRNAi L3 wing disc stained with anti-Bbg (A) and Sqh-GFP (endogenous signal, A′). (B and B′) Pouch of en-Gal4, UAS:RFP, sqh-GFP L3 wing disc stained with anti-Bbg (B) and Sqh-GFP (endogenous signal, B′), respectively. (C) Ratio of fluorescence intensity of Sqh-GFP in en-Gal4, UAS:RFP, sqh-GFP; UAS-bbgRNAi and en-Gal4, UAS:RFP, sqh-GFP L3 wing discs (six independent discs per genotype). (D) WB of protein extracts isolated from sqh-GFP and sqh-GFP;bbgB211 L3 wing discs, showing a reduction of total and phosphorylated Sqh-GFP in sqh-GFP;bbgB211. Tubulin served as loading control. Antibodies used were anti-Bbg, anti-GFP (for both Sqh-GFP and Phospho-Sqh-GFP), and antitubulin. (E) IP from protein extracts isolated from WT and sqh-GFP L3 wing discs, using an anti-GFP antibody (three biological replicates per condition). Bbg is immunoprecipitated from extracts of Sqh-GFP (two right lanes), but not from WT extracts (two left lanes). The statistical analysis (C) used t test and ANOVA. **, P ≤ 0.01. Error bar shows SD. Bars, 25 µm.