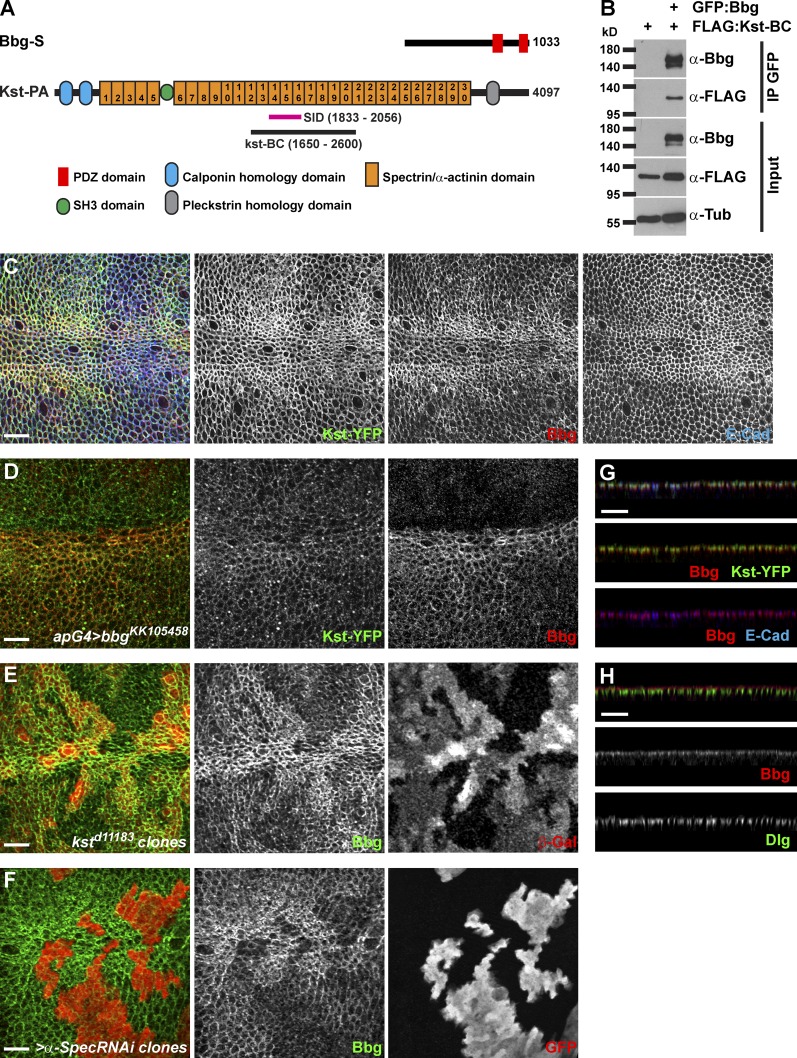
Figure 3.
Bbg binds to the spectrin cytoskeleton. (A) Schematic diagrams of Bbg and the βH-Spectrin Kst structures. In pink is shown the minimal overlap of the Kst Y2H prey clones interacting with Bbg (SID). In black is shown the newly generated Kst-BC fragment used in coIP experiments in B. (B) The Kst-BC fragment coimmunoprecipitates with Bbg. Bottom: Western blot of cleared lysates from cells expressing either FLAG alone (negative control) or FLAG-tagged Kst-BC with GFP-tagged Bbg. Top: Western blot after immunoprecipitation (IP) with anti-GFP. FLAG-tagged Kst-BC was detected only when GFP-Bbg was present. (C) In larval wing imaginal disc epithelial cells, Bbg (red) colocalized with Kst (green). Apical cell junctions were revealed by E-Cad (blue). Note that both Bbg and Kst were found in a cortical ring just inside the membrane-bound belt of E-Cad. (D) The RNAi-mediated depletion of bbg in dorsal wing disc cells using the apterous-Gal4 driver (apG4; top part of the image) did not affect the localization of Kst (Kst-YFP; green, middle), whereas Bbg (red, right) was completely absent. (E and F) In kst mutant clones marked by the absence of β-Gal (E; red, right) or in clones expressing an α-Spec RNAi transgene marked by the presence of GFP (F; red, right), Bbg (green, middle) was only weakly recruited to the cortex. (G) Z section corresponding with the image in C and showing the overlap between Bbg (red, middle and bottom) and Kst-YFP (green, middle) or E-Cad (blue, bottom). (H) Z section showing that Bbg (red, middle) was localized apically to the septate junctions marker Dlg (green, bottom). Bars, 10 µm.