Figure 1.
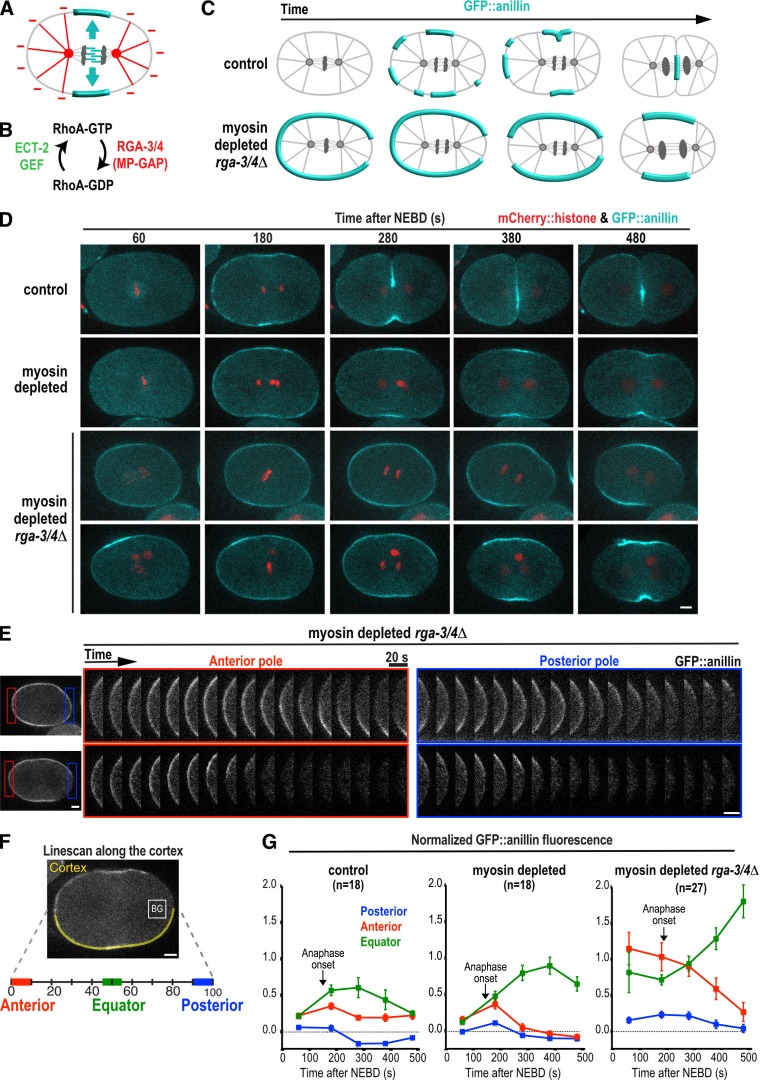
Contractile ring proteins are actively cleared from the polar cortex after anaphase onset. (A) The spindle provides a stimulatory signal that promotes the equatorial accumulation of contractile ring proteins (cyan) and an inhibitory signal that prevents their accumulation on the polar cortex (red). (B) During mitosis, RhoA is activated by the ECT-2 GEF and inactivated by MP-GAP (RGA-3/4 in C. elegans). (C) Schematics illustrating the localization of the contractile ring marker GFP::anillin during the first division of control (top) and myosin-depleted rga-3/4Δ (bottom) embryos. (D) Representative time-lapse series of the first division of control (n = 9, top row) and myosin-depleted (n = 9, second row) embryos expressing GFP::anillin (cyan) and mCherry::histone (red). Two representative myosin-depleted rga-3/4Δ embryos are shown (bottom two rows): one in which GFP::anillin is evenly distributed around the cortex before anaphase onset (n = 6/14; third row), and one in which GFP::anillin is absent from the posterior cortex before anaphase onset (n = 8/14; bottom row). Time is seconds after NEBD. (E) Kymographs of the anterior and posterior poles in the myosin-depleted rga-3/4Δ embryos shown in D beginning 180 s after NEBD. (F) GFP::anillin fluorescence on the anterior, posterior, and equatorial cortex was quantified as shown after subtraction of mean cytoplasmic background (BG, measured in box). The mean GFP::anillin fluorescence in each region was normalized by dividing by the mean intensity at the anterior pole in myosin-depleted rga-3/4Δ embryos 180 s after NEBD. (G) Normalized cortical GFP::anillin fluorescence is plotted for the anterior, posterior, and equatorial cortex in control, myosin-depleted, and myosin-depleted rga-3/4Δ embryos. Arrows mark the mean point of anaphase onset for each condition. Error bars are SEM; n = number of linescans. Bars, 5 µm.