Abstract
Background:
Laparoscopic left lateral hepatic sectionectomy (LLLHS) has been widely accepted because of the benefits of minimally invasive surgery. We aimed to assess the benefits and drawbacks of left lateral sectionectomy (of segments II/III) compared with laparoscopic and open approaches.
Methods:
Relevant literature was searched using the PubMed, Embase, Cochrane, and Ovid Medline databases. We calculated odds ratios or mean differences with 95% confidence intervals (CIs) for fixed-effects and random-effects models.
Results:
The meta-analysis included 14 trials involving 685 patients. There were no statistically significant differences between LLLHS and open LLHS (OLLHS) regarding analgesia (P = .31), pedicle clamping (P = .70), operative time (P = .54), hospital expenses (P = .64), postoperative alanine aminotransferase levels (P = .57), resection margin (95% CI –3.02–4.28; P = .73), or tumor recurrence (95% CI 0.51–3.05; P = .62). However, the LLLHS group showed significantly better results regarding blood transfusion (95% CI 0.14–0.73; P = .007), blood loss (95% CI −140.95 to −67.23; P <.001), total morbidity (95% CI 0.24–0.56; P <.01), and hospital stay (95% CI –3.84 to –2.31; P <.001) than the OLLHS group.
Conclusion:
LLLHS has an advantage in the hospital stay, blood loss, and total morbidity. It is an ideal method for LLHS surgery.
Keywords: hepatectomy, laparoscopic, left lateral hepatic sectionectomy
1. Introduction
The conventional approach of open liver resection is considered an effective treatment option.[1,2] Laparoscopic liver resection was first attempted in 1992.[3] Currently, with the rapid development of hepatic surgery and laparoscopic surgical techniques, laparoscopic liver resection is gaining popularity.[4–9] Left lateral hepatic sectionectomy (LLHS) represents the most common procedure in many laparoscopic series worldwide and is particularly suitable for minimally invasive surgery because the left lateral lobe is more likely to be exposed than other segments during surgery owing to its small volume in the abdominal cavity, relatively independent vascular branching and clear vasculature gradation.
Open left lateral hepatic sectionectomy (OLLHS) is performed through a abdominal incision. The surgery is performed by resecting lesions using open surgical instruments, and hemostasis is achieved by intraoperative pedicle clamping to control the liver blood flow. However, the treatment leaves a noticeable scar and the recovery is painful and slow. The ideal treatment for liver surgery should be simple, minimally invasive, and reliable. Additionally, it should allow fast functional recovery, cause minimal pain, and be affordable for the patients. Laparoscopic left lateral hepatic sectionectomy (LLLHS) fulfils those requirements.[10] However, in the past, important LLLHS drawbacks were that the surgery was relatively complex, demanded a long time, and showed higher incidence of postoperative complications such as bleeding and gas embolism compared to OLLHS.[11] Thus, until more studies were performed, there was no conclusion about which technique was superior.
Recently, some scholars proposed laparoscopy as the gold standard for LLHS.[12,13] However, this proposal was based on a single-center study. The limited availability of clinical data and the lack of multicenter studies make it difficult to support this view. Furthermore, no clear guidelines for the indications of the laparoscopic approach are available. The purpose of this study was to perform a pooled analysis of published data on both laparoscopic and OLLHS.
2. Methods
2.1. Literature search
A systematic search was carried out using PubMed, Embase, Cochrane Database of Systematic Reviews, and Ovid Medline with the following keywords: hepatectomy, left lateral liver resection, segments II and III, laparoscopic hepatectomy, and laparoscopic versus open. The search was conducted to include articles published between the date of the creation of the electronic resource and May 20, 2016. Additionally, we searched the reference lists of selected papers and systematic reviews for potentially relevant studies missed by the original search. Two reviewers independently selected the eligible studies. Disagreement on article inclusion between the 2 reviewers was resolved by discussion with a third reviewer. This study protocol followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement and the Cochrane Handbook for Diagnostic Test Accuracy Reviews. Institutional review board approval and patient consent were waived because of the review nature of this study. The qualities of studies were assessed using Newcastle–Ottawa scale (NOS).[14] A star system of the NOS (range, 0–9 stars) has been developed for the evaluation (Table 1). The study was approved by the Second Affiliated Hospital of Nanchang University Ethics Committee.
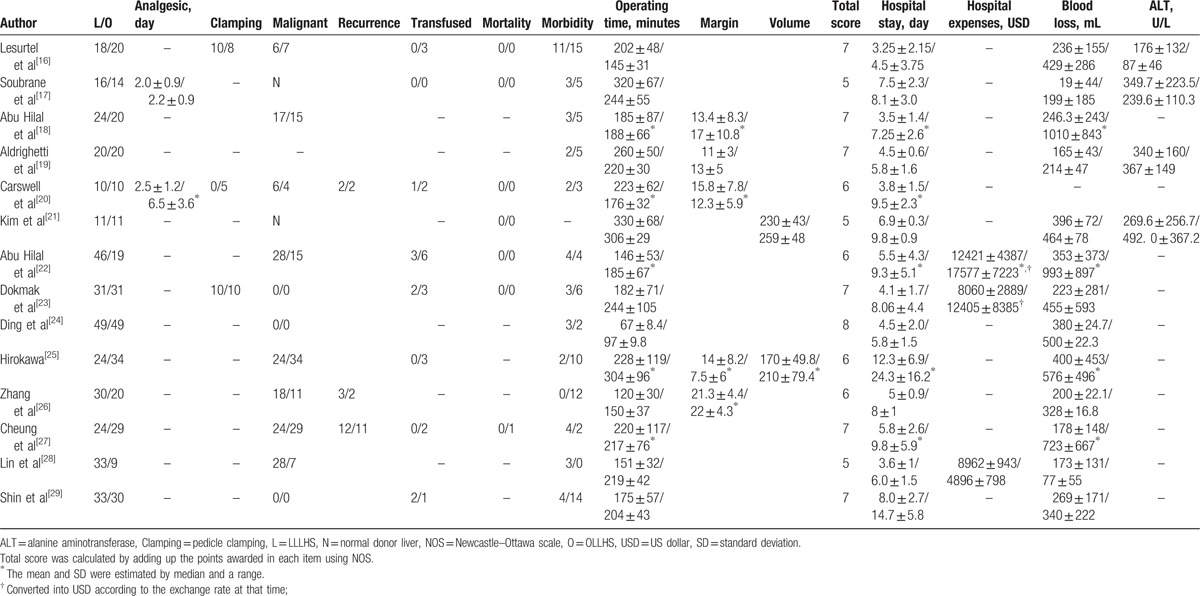
Table 1.
Description of included trials.
2.2. Inclusion and exclusion criteria
All case–control studies in which LLLHS were compared with OLLHS were selected. Studies were included if they involved patients with no requirement of additional procedures, no history of upper laparotomy and who had the LLHS (including malignant, benign and normal results). Additionally, included studies must have reported data on analgesia, pedicle clamping, malignancy, blood transfusion, mortality, total morbidity, operative time (minutes), length of hospital stay (days), hospital expense (dollars), blood loss (milliliters), and/or postoperative alanine aminotransferase (ALT) levels.
The total morbidity included bile leakage, bleeding, ascites, pneumonia, incisional hernia, abscess, urine infection, ulcer, and pulmonary embolus.
The exclusion criteria were articles not reporting outcomes, editorials, review articles, and animal studies. Neither authorship nor publisher information influenced our which articles were included.
2.3. Statistical analysis
The analyses were performed using Review Manager version 5.1 (RevMan, Cochrane Collaboration, Oxford, UK). The results of this meta-analysis are expressed as the odds ratios (ORs) for dichotomous data and mean differences (MDs) for continuous data, with 95% confidence intervals (CIs) for both. The inverse variance method was used for continuous variables, whereas the Mantel–Haenszel method was used for dichotomous variables. Statistical heterogeneity was evaluated by χ2 test. P <.05 was considered significant. If heterogeneity was significant, we used the random-effects model. Otherwise, we used the fixed-effects model. If data were reported as median and range rather than mean and standard deviation (SD), the mean and SD were estimated as described previously.[15]
3. Results
3.1. Study selection and characteristics
Figure 1 illustrates the search process and the final selection of relevant studies. We analyzed 14 trials,[16–29] involving 685 patients, that met the criteria (Table 1).
Figure 1.
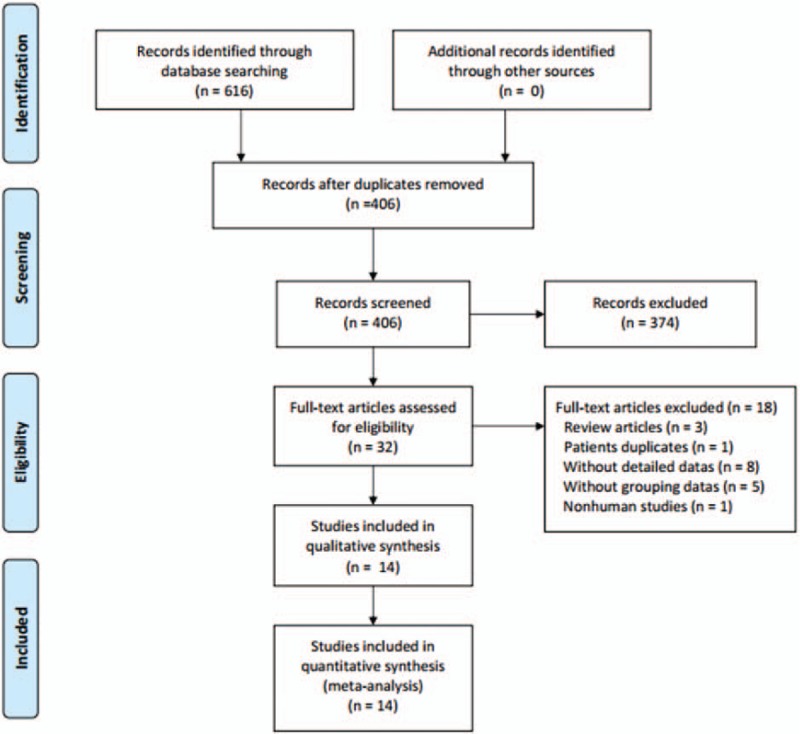
Flow diagram of study selection.
3.2. Operative time
Information on operative time was provided in all 14 trials. There was no statistically significant difference between the LLLHS and OLLHS groups (MD −6.46, 95% CI −27.25–14.33; P = . 54; Fig. 2A).
Figure 2.
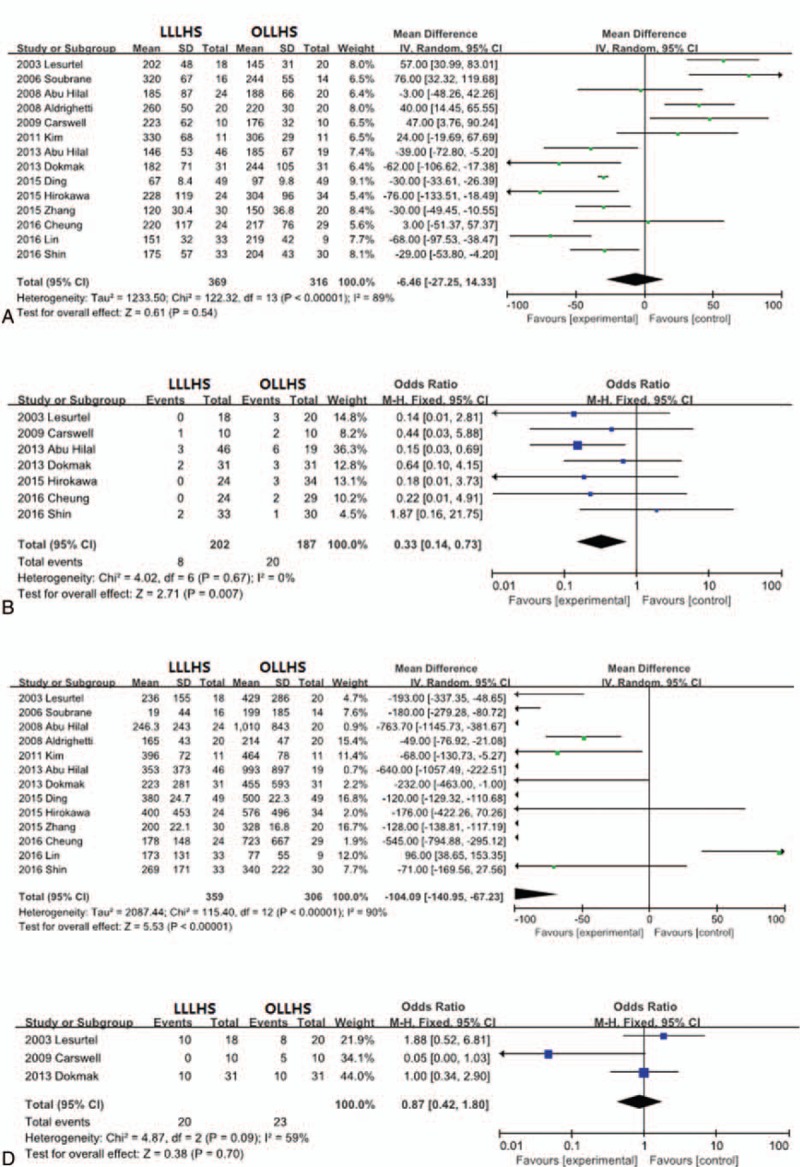
Forest plot of meta-analysis showing the random-effects model of the mean differences for operative time. (A) Fixed-effects model of the odds ratios for blood transfusion, (B) random-effects model of the mean differences for blood loss, (C) and fixed-effects model of the odds ratios for pedicle clamping (D).
3.3. Blood transfusion, blood loss, and pedicle clamping
Thirteen trials reported information on blood loss, 7 trials reported transfusion information, and 3 trials included information on the need for pedicle clamping. Eight of 202 patients (3.96%) and 20 of 187 patients (10.7%) required blood transfusion in the LLLHS and OLLHS groups, respectively. The need for blood transfusion (OR 0.33, 95% CI 0.14–0.73; P = .007; Fig. 2B) and blood loss (MD –104.09, 95% CI −140.95 to −67.23; P <.001; Fig. 2C) in the LLLHS group were significantly lower than in the OLLHS group. Pedicle clamping was performed in 20 of 59 patients (33.9%) in the LLLHS group and 23 of 51 patients (45.1%) in the OLLHS group. The number of patients requiring pedicle clamping did not differ significantly between the 2 groups. (OR 0.87, 95% CI 0.42–1.80; P = .70; Fig. 2D).
3.4. Hospital stay
The length of the hospital stay was evaluated in 14 studies. The patients in the LLLHS group had a shorter hospital stay than the OLLHS group by an average of 3.08 days (95% CI –3.84 to –2.31; P <.001; Fig. 3A).
Figure 3.
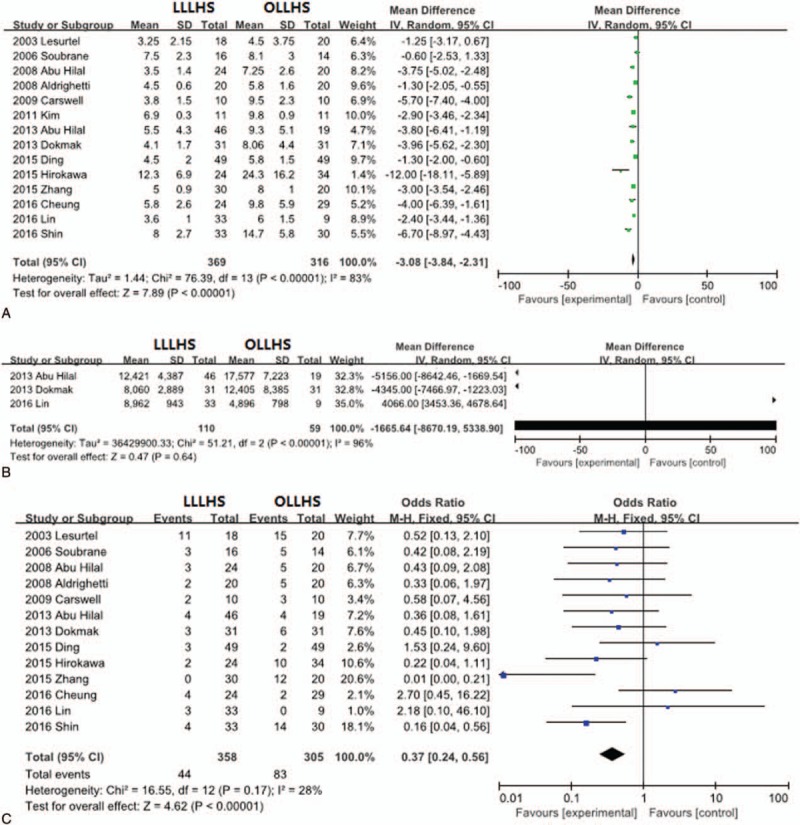
Forest plot of meta-analysis showing the random-effects model of the mean differences for length of hospital stay (A) and hospitalization charges (B) and the fixed-effects model of odds ratios for total morbidity (C).
3.5. Hospitalization costs
Information on hospitalization costs was reported in only 3 trials. The patients in the LLLHS group incurred lower hospitalization costs than the OLLHS group by an average of $1665.64 (95% CI –8670.19–5338.90; Fig. 3B), but this difference was not significant (P = .64). Furthermore, there was substantial heterogeneity in hospitalization costs among the included studies (P <.001).
3.6. Total morbidity
Thirteen trials provided information on total morbidity. The occurrence of total morbidity was 12.3% (44 out of 358 patients) and 27.2% (83 out of 305 patients) for the LLLHS and the OLLHS groups, respectively. The total morbidity in the LLLHS group was significantly lower than that in the OLLHS group (OR 0.37, 95% CI 0.24–0.56; P <.001; Fig. 3C).
3.7. Postoperative analgesia and ALT levels
Only 2 trials included information about analgesia, and only 3 trials reported postoperative ALT levels. There were no statistically significant differences in analgesia (MD −1.92, 95% CI −5.63–1.78; P = .31; Fig. 4A) or postoperative ALT levels (MD 28.02, 95% CI −69.44–125.47; P = .57; Fig. 4B) between the LLLHS and OLLHS groups.
Figure 4.
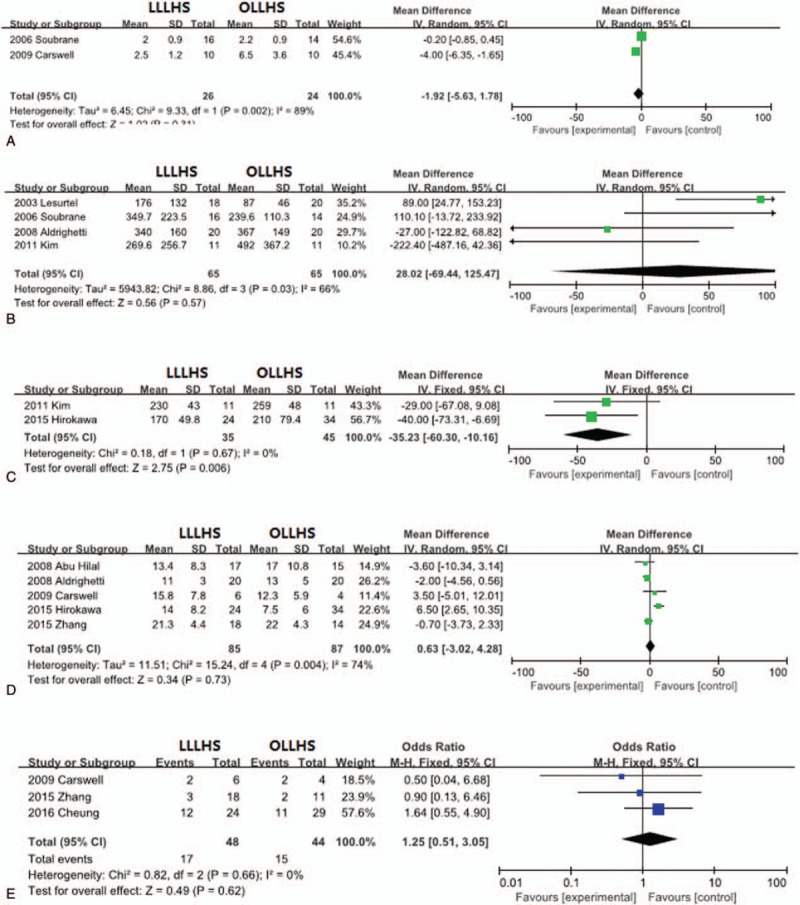
Forest plot of meta-analysis showing the random-effects model of mean differences for length of analgesia, (A) postoperative ALT levels, (B) liver resection volume, (C) resection margin, (D) and tumor recurrence (E). ALT = alanine aminotransferase.
3.8. Liver resection volume
Two trials reported resection liver volume. The resection volume in the OLLHS group was significantly larger than that in the LLLHS group (MD –35.23, 95% CI –60.3 to –10.16; P = .006; Fig. 4C).
3.9. Tumor resection margin and recurrence
The resection margin was evaluated in 5 studies, and tumor recurrence was evaluated in 3 studies. There were no significant differences between the LLLHS and OLLHS groups for the resection margin (MD 0.63, 95% CI –3.02–4.28; P = .73; Fig. 4D) or tumor recurrence (OR 1.25, 95% CI 0.51–3.05; P = .62; Fig. 4E).
3.10. Publication bias
In the present meta-analysis, the funnel plots for blood transfusion (Fig. 5A), blood loss (Fig. 5B), hospital stay (Fig. 5C), total morbidity (Fig. 5D), and liver resection volume (Fig. 5E) showed basic symmetry. No significant publication bias was observed. The results were similar, and the combined results were highly reliable.
Figure 5.
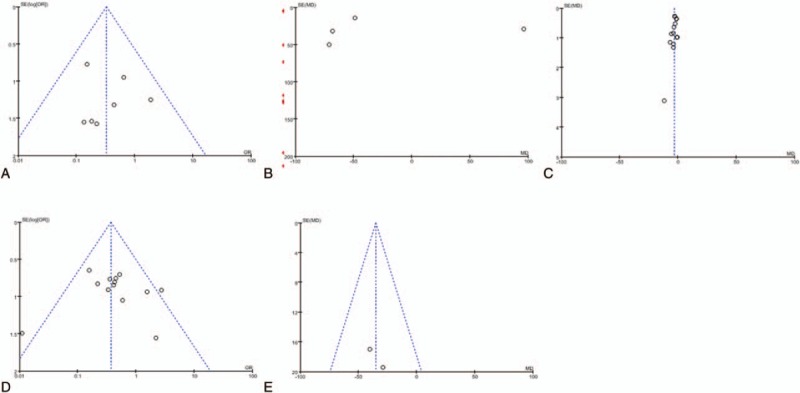
Funnel plots for meta-analyses. (A) Seven articles in the meta-analysis of blood transfusion, (B) 13 articles in the meta-analysis of blood loss, (C) 14 articles in the meta-analysis of hospital stay, (D) 13 articles in the meta-analysis of total morbidity, (E) 2 articles in the meta-analysis of liver resection volume.
4. Discussion
With recent advancements in laparoscopic technology, laparoscopic liver resections can be performed safely with high efficiency and minimal morbidity and mortality.[10,12,30–32] However, laparoscopic liver resections have not gained wide acceptance for the following reasons. First, the liver blood supply comes from 2 different blood vessels, namely the hepatic artery and the portal vein, commonly causing bleeding during the resection. Furthermore, it is difficult to accurately control bleeding by laparoscopy. Second, inadequate surgical field exposure causes hepatic lesions, making it difficult to control hemostasis, increasing the operative time. Third, certain useful techniques typically applied during open hepatectomy are difficult to implement in laparoscopic liver resection, such as vessel hemostasis by applying pressure to the wall, and stitching. Fourth, there are concerns that laparoscopic liver resection may lead to the spread of malignant cells or that the resection margins may be insufficient. Finally, the availability of the equipment needed for laparoscopic liver resection is limited compared with that needed for open liver resection.
The left lateral hepatic lobe has unique anatomical features. It occupies a relatively small volume in the abdomen, in proximity to the abdominal wall. Additionally, it exhibits a relatively simple intrahepatic duct line and clear borders with the surrounding organs. These anatomical features of the left lateral hepatic lobe constitute favorable conditions for LLLHS. Because of these features, it is relatively simple to perform laparoscopic or open hemihepatectomy. In recent years, the number of LLLHSs has increased. The procedure has matured and constitutes a treatment option for liver tumors, hepatic cavernous hemangioma, and intrahepatic bile duct stones, provided that the lesions are located in the left lateral hepatic lobe. It has been further suggested that LLLHS should be the gold standard treatment for left lobe lesions, but such suggestions lack large-sample multicenter clinical evidence to support them. In our work, we considered the operative time, postoperative complications, blood loss, and hospital stay, reported in many individual studies, in order to compare and analyze LLLHS and OLLHS. We found that the LLHS indeed has advantages over the open resection procedure.
A previous study reported shorter operative time for open resection and no significant difference in blood loss between LLLHS and OLLHS groups.[13] However, our results showed that the operative time in the LLLHS group was similar to that in the OLLHS group, whereas the total morbidity rate was lower in the LLLHS group. We believe that these discrepancies in the results are due to 2 reasons. One reason is that the previous work was published in 2011 and only 7 studies were included. Our research is more up to date and included 14 studies. The other reason is that operative time decreased over time, suggesting that surgeons effectively improved their technical skills over time and gained confidence with the surgical field, reflecting the standardization of surgical steps. Additionally, the worldwide use of Endo GIA staplers made the LLLHS simpler and safer.[33]
Occasional bleeding and hepatic vein injuries are the most common hepatectomy risks, regardless of the approach.[12] It is not as easy to control bleeding during laparoscopic hepatic resection as it is during open hepatic resection. Nevertheless, our results showed that the patients in the LLLHS group were less likely to require blood transfusion and experienced less blood loss compared with those in the OLLHS group, whereas the number of patients requiring pedicle clamping did not significantly differ between the 2 groups. Additionally, the total morbidity in the LLLHS group was significantly lower than that in the OLLHS group. There was 1 patient death in the OLLHS group, and no patient death in the LLLHS group. Furthermore, the LLLHS hospitalization costs were $1665.64 lower than those for OLLHL (however, this difference was not statistically significant). There was no statistically significant difference between the 2 groups for postoperative use of analgesics or ALT levels.
The ideal level for left lateral hepatic lobe resection is in the sagittal plane, which passes 1 cm to the left of the incisure of the round ligament of the liver. The major blood vessels through this plane include the upper and inferior branches of the left lateral hepatic portal vein as well as the main and superior left branches of the hepatic vein. They are distributed in the 2 superior thirds of this plane. Therefore, a detailed preoperative evaluation is indispensable. Preoperative computed tomography for vascular exploration, magnetic resonance imaging, or digital subtraction angiography can not only reveal the size of the lesions but also reveal any variations in the blood vessels or the biliary tract.
The central venous pressure is kept between 4 and 6 cm H2O, which represents the optimal intraoperative range for reducing bleeding and hepatic vein reflux.[34] Moreover, the use of the Endo GIA stapler not only greatly reduces operative time but also effectively prevents bleeding. The surgical expertise and instruments used (such as the ultrasonic dissector, argon beam, LigaSure, microwave coagulators, and ultrasound laparoscopic transducer), together with Endo-GIA vascular staplers and suturing techniques, are improving constantly, leading to a rapid, worldwide increase in the popularity of laparoscopic liver resection.
For tumor patients, the concerns regarding insufficient margin resection and tumor rupture, both causing cancer cells to seed through surgical ports, have prevented the widespread application of laparoscopic liver resection.[12] However, we found that there were no statistically significant differences between the LLLHS and OLLHS groups regarding the pathological resection margin or tumor recurrence. To our knowledge, reports of hypercapnia and carbon dioxide gas embolism are very rare.[14–29]
Our study should be interpreted with caution due to its limitations. First, the patient selection process may have been biased. The standards were different for different trials, depending on the technology used, on the expertise level of the surgeons, and on the anatomical conditions of the patients. Moreover, no randomized controlled trials were included in our study. Second, there were insufficient data to allow reliable comparison of the long-term postoperative recurrence rate and survival rate between the LLLHS and OLLHS groups for tumor patients. Third, although we tried to identify all relevant data, potential publication bias was unavoidable; therefore, some relevant data may have been overlooked. Finally, since this study was based only on reports published in English, publication bias could not be completely ruled out.
Consistent with the modern concept of enhanced recovery after surgery, the results show that although LLLHS requires higher laparoscopic skills and has a longer learning curve,[30] it was worth recommending for treating left lobe lesions. Laparoscopic technology was applied for the first time in cholecystectomy in the 1980s,[35] and now laparoscopic cholecystectomy has fully replaced open cholecystectomy. Similar to laparoscopic cholecystectomy, LLLHS is expected to become the gold standard for the treatment of left hepatic lobe lesions.
Footnotes
Abbreviations: ALT = alanine aminotransferase, CIs = confidence intervals, LLHS = left lateral hepatic sectionectomy, LLLHS = laparoscopic left lateral hepatic sectionectomy, MDs = mean differences, NOS = Newcastle–Ottawa scale, OLLHS = open left lateral hepatic sectionectomy, ORs = odds ratios, PRISMA = Systematic Reviews and Meta-Analyses, SD = standard deviation.
ZL and HD contributed equally to this work.
Funding: This work was supported by the Natural Science Foundation of China (no. 81760514) and Youth Science Fund of Jiangxi Provincial Science and Technology Department (no. 20161BAB215252).
The authors have no conflicts of interest to disclose.
References
- [1].Montalti R, Berardi G, Laurent S, et al. Laparoscopic liver resection compared to open approach in patients with colorectal liver metastases improves further resectability: oncological outcomes of a case-control matched-pairs analysis. Eur J Surg Oncol 2014;40:536–44. [DOI] [PubMed] [Google Scholar]
- [2].Buell JF, Rosen S, Yoshida A, et al. Hepatic resection: effective treatment for primary and secondary tumors. Surgery 2000;128:686–93. [DOI] [PubMed] [Google Scholar]
- [3].Topal B, Fieuws S, Aerts R, et al. Laparoscopic versus open liver resection of hepatic neoplasms: comparative analysis of short-term results. Surg Endosc 2008;22:2208–13. [DOI] [PubMed] [Google Scholar]
- [4].Nachmany I, Pencovich N, Zohar N, et al. Laparoscopic versus open liver resection for metastatic colorectal cancer. Eur J Surg Oncol 2015;41:1615–20. [DOI] [PubMed] [Google Scholar]
- [5].Vibert E, Perniceni T, Levard H, et al. Laparoscopic liver resection. Br J Surg 2006;93:67–72. [DOI] [PubMed] [Google Scholar]
- [6].Gigot JF, Glineur D, Santiago Azagra J, et al. Laparoscopic liver resection for malignant liver tumors: preliminary results of a multicenter European study. Ann Surg 2002;236:90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Calise F, Giuliani A, Sodano L, et al. Segmentectomy: is minimally invasive surgery going to change a liver dogma? Updates Surg 2015;67:111–5. [DOI] [PubMed] [Google Scholar]
- [8].Giuliani A, Aldrighetti L, Di Benedetto F, et al. Total abdominal approach for postero-superior segments (7,8) in laparoscopic liver surgery: a multicentric experience. Updates Surg 2015;67:169–75. [DOI] [PubMed] [Google Scholar]
- [9].Giuliani A, Migliaccio C, Ceriello A, et al. Laparoscopic vs open surgery for treating benign liver lesions: assessing quality of life in the first year after surgery. Updates Surg 2014;66:127–33. [DOI] [PubMed] [Google Scholar]
- [10].Vanounou T, Steel JL, Nguyen KT, et al. Comparing the clinical and economic impact of laparoscopic versus open liver resection. Ann Surg Oncol 2010;17:998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Biertho L, Waage A, Gagner M. Laparoscopic hepatectomy. Ann Chir 2002;127:164–70. [DOI] [PubMed] [Google Scholar]
- [12].Azagra JS, Goergen M, Brondello S, et al. Laparoscopic liver sectionectomy 2 and 3 (LLS 2 and 3): towards the “gold standard”. J Hepatobiliary Pancreat Surg 2009;16:422–6. [DOI] [PubMed] [Google Scholar]
- [13].Rao A, Rao G, Ahmed I. Laparoscopic left lateral liver resection should be a standard operation. Surg Endosc 2011;25:1603–10. [DOI] [PubMed] [Google Scholar]
- [14].Cota GF, de Sousa MR, Fereguetti TO, et al. Efficacy of anti-leishmania therapy in visceral leishmaniasis among HIV infected patients: a systematic review with indirect comparison. PLoS Negl Trop Dis 2013;7:e2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lesurtel M, Cherqui D, Laurent A, et al. Laparoscopic versus open left lateral hepatic lobectomy: a case-control study. J Am Coll Surg 2003;196:236–42. [DOI] [PubMed] [Google Scholar]
- [17].Soubrane O, Cherqui D, Scatton O, et al. Laparoscopic left lateral sectionectomy in living donors: safety and reproducibility of the technique in a single center. Ann Surg 2006;244:815–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Abu Hilal M, McPhail MJ, Zeidan B, et al. Laparoscopic versus open left lateral hepatic sectionectomy: a comparative study. Eur J Surg Oncol 2008;34:1285–8. [DOI] [PubMed] [Google Scholar]
- [19].Aldrighetti L, Pulitanò C, Catena M, et al. A prospective evaluation of laparoscopic versus open left lateral hepatic sectionectomy. J Gastrointest Surg 2008;12:457–62. [DOI] [PubMed] [Google Scholar]
- [20].Carswell KA, Sagias FG, Murgatroyd B, et al. Laparoscopic versus open left lateral segmentectomy. BMC Surg 2009;9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kim KH, Jung DH, Park KM, et al. Comparison of open and laparoscopic live donor left lateral sectionectomy. Br J Surg 2011;98:1302–8. [DOI] [PubMed] [Google Scholar]
- [22].Abu Hilal M, Di Fabio F, Syed S, et al. Assessment of the financial implications for laparoscopic liver surgery: a single-centre UK cost analysis for minor and major hepatectomy. Surg Endosc 2013;27:2542–50. [DOI] [PubMed] [Google Scholar]
- [23].Dokmak S, Raut V, Aussilhou B, et al. Laparoscopic left lateral resection is the gold standard for benign liver lesions: a case-control study. HPB (Oxford) 2014;16:183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ding G, Cai W, Qin M. Pure laparoscopic versus open liver resection in treatment of hepatolithiasis within the left lobes: a randomized trial study. Surg Laparosc Endosc Percutan Tech 2015;25:392–4. [DOI] [PubMed] [Google Scholar]
- [25].Hirokawa F, Hayashi M, Miyamoto Y, et al. Short- and long-term outcomes of laparoscopic versus open hepatectomy for small malignant liver tumors: a single-center experience. Surg Endosc 2015;29:458–65. [DOI] [PubMed] [Google Scholar]
- [26].Zhang Y, Chen XM, Sun DL. Comparison of laparoscopic versus open left lateral segmentectomy. Int J Clin Exp Med 2015;8:904–9. [PMC free article] [PubMed] [Google Scholar]
- [27].Cheung TT, Poon RT, Dai WC, et al. Pure laparoscopic versus open left lateral sectionectomy for hepatocellular carcinoma: a single-center experience. World J Surg 2016;40:198–205. [DOI] [PubMed] [Google Scholar]
- [28].Lin TL, Alikhanov R, Kuo SC, et al. Less cost by using hanging maneuver and Pringle maneuver in left lateral hepatectomy through small laparotomy wound-experience of Southern Taiwan. World J Surg Oncol 2016;14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shin YC, Jang JY, Kang MJ, et al. Comparison of laparoscopic versus open left-sided hepatectomy for intrahepatic duct stones. Surg Endosc 2016;30:259–65. [DOI] [PubMed] [Google Scholar]
- [30].Ratti F, Barkhatov LI, Tomassini F, et al. Learning curve of self-taught laparoscopic liver surgeons in left lateral sectionectomy: results from an international multi-institutional analysis on 245 cases. Surg Endosc 2015;30:3618–29. [DOI] [PubMed] [Google Scholar]
- [31].Abu Hilal M, Pearce NW. Laparoscopic left lateral liver sectionectomy: a safe, efficient, reproducible technique. Dig Surg 2008;25:305–8. [DOI] [PubMed] [Google Scholar]
- [32].Polignano FM, Quyn AJ, de Figueiredo RS, et al. Laparoscopic versus open liver segmentectomy: prospective, case-matched, intention-to-treat analysis of clinical outcomes and cost effectiveness. Surg Endosc 2008;22:2564–70. [DOI] [PubMed] [Google Scholar]
- [33].Schemmer P, Friess H, Dervenis C, et al. The use of endo-GIA vascular staplers in liver surgery and their potential benefit: a review. Dig Surg 2007;24:300–5. [DOI] [PubMed] [Google Scholar]
- [34].Montorsi M, Santambrogio R, Bianchi P, et al. Perspectives and draw backs of minimally invasive surgery for hepatocellular carcinoma. Hepatogastroenterology 2002;49:56–61. [PubMed] [Google Scholar]
- [35].Litynski GS. Profiles in laparoscopy: Mouret, Dubois, and Perissat: the laparoscopic breakthrough in Europe (1987–1988). JSLS 1999;3:163–7. [PMC free article] [PubMed] [Google Scholar]


