Abstract
Background
The aim of this study is to estimate the reduction in new HIV infections and resultant cost outcomes of providing antiretroviral treatment (ART) through Australia's ‘universal access’ health scheme to all temporary residents with HIV infection living legally in Australia, but currently deemed ineligible to access subsidised ART via this scheme.
Methods
A mathematical model to estimate the number of new HIV infections averted and the associated lifetime costs over 5 years if all HIV-positive temporary residents in Australia had access to ART and subsidised medical care was developed. Input data came from a cohort of 180 HIV-positive temporary residents living in Australia who are receiving free ART donated by pharmaceutical companies for up to 4 years.
Results
Expanding ART access to an estimated total 450 HIV+ temporary residents in Australia for 5 years could avert 80 new infections. The model estimated the total median discounted (5%) cost for ART and associated care to be A$36 million, while the total savings in lifetime-discounted costs for the new infections averted was A$22 million.
Conclusions
It is estimated that expanded access to ART for all HIV-positive temporary residents in Australia will substantially reduce HIV transmission to their sexual partners at little additional cost. In the context of Australia's National HIV strategy and Australia's endorsement of global goals to provide universal access to ART for all people with HIV, this is an important measure to remove inequities in the provision of HIV-related treatment and care.
Additional keywords: antiretroviral therapy, Australia, HIV prevention, mathematical modelling, migrants, treatment access
Introduction
Effective antiretroviral treatment (ART) for HIV-positive (HIV+) individuals results in viral suppression leading to long-term disease remission, prolonged survival and the prevention of HIV transmission.1–8 Providing ART to infected individuals is a key prevention strategy and a crucial component of the HIV response internationally.9,10 Maintaining an undetectable viral load, however, requires regular access to care and treatment, with patients adhering to their treatment regimen.
The Australian government provides fully subsidised ART to HIV+ patients eligible for a Medicare card through the Pharmaceutical Benefits Scheme (PBS).11. Until they apply for residency, HIV+ temporary residents living in Australia are ineligible for a Medicare card and cannot access subsidised ART. Thus, most HIV+ temporary residents have limited options for accessing treatment and must pay the full cost of ART medications (with first-line regimens costing ∼A$10 500 per year12), source ART from their country of origin or import generic drugs from overseas suppliers. Such barriers prevent HIV+ temporary residents achieving optimal health outcomes and increase the risk of transmission to their sexual partners.13,14
The Australian HIV Observational Database (AHOD) Temporary Residents Access Study (ATRAS) commenced in November 2011 and followed 180 HIV+ temporary residents in clinical and financial need of subsidised ART for 4 years.14,15 At enrolment, 63% of ATRAS patients were receiving ART from alternative sources, with only 70% having a suppressed viral load (<50 copies mL-1 ); suggesting many were receiving suboptimal treatment. This percentage increased to 85% at 12-months follow up and to 96% at 24-months follow up. ATRAS only provided ART to the end of 2015 to a proportion of all HIV+ temporary residents who were ineligible for Medicare in Australia.14,15 Using data from ATRAS, we estimated the effect and potential costs of providing ART to all HIV+ temporary residents in Australia.
Methods
We used a risk equation model to estimate the annual number of new infections over the next 5 years in sexual partners of the estimated 400-500 HIV+ temporary residents currently living in Australia.14,15 The calculations used parameter values based on the characteristics of the ATRAS cohort patients at enrolment, at 12-months follow up and at 24-months follow up.14,15 For projections beyond the years of available data, we used the most recent data value available. For each parameter, we specified a range of values and then sampled 1000 parameter sets. All model parameter values with justifications are provided in Table 1. We repeated the calculations for each parameter set 20 times to account for stochastic variations. Results were obtained using summary statistics from all simulations. Total costs are in AUD and rounded to the nearest A$10 000. We provide a detailed description of the model equations, assumptions, and input parameter values and reproducible code in an online repository.20 All the analyses and results were generated using R version 3.1.2 and associated packages.21
Table 1. Calculation input parameter ranges.
Footnotes provide justifications for these parameter ranges. The simulations used for the calculations take samples from these ranges assuming a uniform distribution. PLWHIV, people living with HIV; MSM, men who have sex with men; ART, antiretroviral treatment
| Parameter | Description | Range | Footnote |
|---|---|---|---|
| Demographic | |||
| N(0) | Overall population size in initial year | [400–500] | 1 |
| p | Multiplicative change in annual population | [0.98–1.02] per year | 1 |
| pm | Proportion of Medicare-ineligible population of PLWHIV who are MSM | [0.4–0.6] | 1 |
| pn | Proportion of Medicare-ineligible population of PLWHIV who are non-MSM | Given by 1–pm | 1 |
| Clinical | |||
| θ | Proportion of population taking ART | 63% at enrolment and 95% after 12 months ±5% | 2 |
| ψ | Proportion of population taking ART with undetectable viral load | 70% at enrolment, 88% after 12 months, and 96% after 24 months ±5% | 2 |
| HIV transmission | |||
| βn | Annual probability an untreated non-MSM transmits HIV to another person | [0.0485–0.0808] | 3 |
| βm | Annual probability an untreated MSM transmits HIV to another person | [0.0771–0.1285] | 3 |
| εART | Efficacy of ART in preventing HIV transmission if virus is suppressed | [0.9–0.99] | 4 |
| Treatment costs | |||
| cART | Average annual undiscounted cost of providing care and ART to Medicare ineligibles (including medical care costs) | [A$10 000–A$20 000] | 5 |
| clife | Average undiscounted lifetime cost of providing care and ART post infection (including medical care costs) | [A$761 800–A$1 269 700] | 6 |
| rdisc | Discounting rate | 5% | 7 |
| tART | Average time between infection and initiating ART | [4–5] years | 7 |
The 2013 Australian HIV Observational Database (AHOD) Temporary Residents Access Study (ATRAS) report estimates there are 450 Medicare ineligible people living with HIV (PLWHIV) in Australia.15 We assume a range in the population between 400 and 500 PLWHIV, with the potential for only a small change in population size over time. In the population of 180 at enrolment, 89 (49.4%) males attributed their HIV infection to MSM exposure.15 Assuming this reflects the demographic distribution over time, we assume 40–60% of the population consists of MSM with the remainder being non-MSM.
At enrolment, 62.8% of ATRAS patients were already receiving antiretroviral treatment (ART), with 71.8% having an undetectable viral load.15 After enrolment, all patients were put onto ART, resulting in 87% having an undetectable viral load at 12 months and 96% having an undetectable viral load at 24 months. Based on the ATRAS data, we assume the percentage of temporary residents on ART increases from 70% to 95%, with a range of ±5%, with the proportion with undetectable virus increasing from 70% to 96% over 2 years, with a range of ±5%.
These values were calculated using data for the overall population of PLWHIV in Australia and Equation 2 in the main text. In 2013, there were an estimated 26 640 PLWHIV in Australia, and 912 new infections16 of which ∼75% are attributed to homosexual contact.17 According to the 2013 estimates for the HIV treatment cascade in Australia, ∼75% of MSM living with HIV17 and 55% of non-MSM living with HIV are taking ART17 respectively. In both MSM and non-MSM taking ART, ∼90% have an undetectable viral load.15,17 Putting these values into Equation 2 in the main text and assuming ±25% uncertainty produces the values of βn and βm.
We assume those with viral suppression have a 90–99% reduction in transmission to their sexual partners. This assumption is in line with the results from the HPTN-052 trial for those with detectable drug levels7 and the recent PARTNER study, which recorded zero HIV transmissions from 1166 HIV+ people to their sexual partners (though the upper 95% confidence interval was 0.84, 0.88, and 0.97 per 100 couple-years for MSM, heterosexual males and heterosexual females respectively).18
At enrolment, 83% of the ATRAS cohort on ART are taking Tenofovir/Emtrcitabine (Truvada) as the ‘backbone’ of their regime. This means most of those on treatment are taking first-line drugs. For this analysis, we assume all patients are on and remain on first-line ART over the period of analysis and undertake annual monitoring of their infection. From Schneider et al, the average annual cost of first-line drugs in Australia is A$10685 (A$6945-A$14 424).12 Estimated annual medical costs for people living with HIV were also estimated in Schneider et al by CD4 count. Annual medical care and infection monitoring for HIV+ people with CD4 >500 cells μ L-1 , CD4 350–499 cells μ L-1 , CD4 >200-349 cells μ L-1 and CD4 <200 cells μ L-1 was estimated to cost A$3097, A$4402, A$4762 and A$7883 respectively. In recent years, patients in the Australian HIV Observational Database (AHOD) cohort have initiated ART at ∼350 cells μ L-1 . We therefore assumed all patients have a CD4 count >200 cells/μL and the associated annual monitoring costs to range from A$3000 to A$5000. Using these values, we assumed a range in the annual ART cost (including medical care) of A$10 000 to A$20 000.
If a partner of a temporary resident becomes infected with HIV, then they will eventually require care and treatment while they are living in Australia. As we are not tracking their infection progression in this analysis, we used an estimate for the lifetime cost of providing ART. An analysis of the life expectancy of PLWHIV in Australia, given currently available antiretroviral treatments, suggests someone starting treatment in their twenties will be taking ART for ∼40 years,19 spending ∼9 years on first-line drugs, ∼14 years on second-line drugs, ∼3 years on third-line drugs, and the remainder of the time on higher classes of drug. Using the cost estimates from the study by Schneider et al, 12we assume the annual costs of proving each line of drugs is A$10 685 for first-line drugs, A$19 364 for second-line drugs, A$31411 for third-line drugs and A$28 162 for fourth and higher lines of drugs. Multiplying the values for each drug class and summing the resulting values produces the undiscounted treatment cost. To account for all uncertainties in the time patients are on each treatment class and drug costs, we assumed a range of ±25% in the overall undiscounted cost. Finally, we added the annual monitoring cost ranging from A$3000 to A$5000, as described in footnote 5 to produce the undiscounted cost presented here.
To discount future costs of providing ART to temporary residents and to those who become infected, we applied a discount rate of 5% from the year of enrolment in ATRAS for all treatment costs. For discounting purposes, we included the time between infection and initiating ART; we estimated this from data on the CD4 count at initiating therapy and estimates for the rate of CD4 decline. In recent years, participants in the AHOD cohort have initiated ART at ∼350 cells μL-1;15 it is estimated that it takes 4.4 years for a person to reach this CD4 count post infection.16 We therefore assume a range of 4–5 years for the time between infection and ART initiation.
Model population
For this analysis, we assumed the population of HIV+ temporary residents in Australia have the characteristics of people in ATRAS.14,15 We split the overall population into a male group of men who have sex with men (MSM) (which, for the purposes of this analysis, we assume are exclusively homosexual) and into a non-MSM group consisting of females and heterosexual males. We assumed the MSM population made up 40-60% of the overall HIV+ temporary resident population, as reported in ATRAS.14,15 We used this compartmentalisation of the population because partners of MSM are likely to be at higher risk of infection (we did not consider population differences in treatment coverage and adherence).
Number of new infections caused byto HIV+ temporary residents
To estimate new infections due to HIV+ temporary residents, we first calculated the probability of HIV transmission over time.20 Our calculation incorporates the annual probability for an untreated HIV+ person to transmit HIV to another person (β), the proportion of the population taking ART (θ), the proportion taking ART with an undetectable viral load (ψ), and the efficacy of viral suppression on HIV transmission (εART; assumed to be 90–99% 6–8 ); this is given by the equation:
| (1) |
We used 2011-15 ATRAS data for the proportion of HIV+ temporary residents taking ART and the corresponding proportion with an undetectable viral load. By using the calculated probability for each year (Equation 1), we then estimated the number of new infections each year (I) using a binomial distribution and the estimated population size for HIV+ temporary residents (N); that is, I∼binom(N,β′). From Equation 1, for large N and small β′, this is approximately equal to:
| (2) |
We estimated the annual probability that an untreated HIV+ person transmits HIV to another person (β) using a weighted average of the separate probabilities for HIV+ temporary resident MSM and non-MSM. As the number of new infections for temporary residents is unknown, we assumed the transmission probability for untreated HIV+ temporary residents equals the transmission probability for the overall untreated HIV+ population in Australia. Substituting estimates for the overall Australian population obtained from the 2014 HIV care and diagnosis cascade (with a 25% uncertainty range)22,16 into Equation 2, and using annual notifications in the overall population in place of new infections (this assumption has been validated in previous modelling studies),23, we obtained a range in the transmission risk for non-MSM of 0.049–0.081 per year and 0.077–0.128 per year for MSM (Table 1). This estimate for β implicitly includes behaviour affecting HIV transmission and the impact of current prevention programs. Our analysis then assessed the impact of ART and viral suppression, in addition to this background transmission.
The ATRAS cohort only provides clinical data and does not report behavioural data or sexual partner characteristics relevant to HIV transmission. Due to this lack of data, we were unable to develop a more detailed model of HIV transmission for HIV+ temporary residents. Thus, our analysis did not include additional infections due to transmission from newly infected partners (unless they are also temporary residents in which case they would become part of the HIV+ temporary resident population). Only infections directly attributed to HIV+ temporary residents were included. We also assumed HIV+ temporary residents on ART, but with unsuppressed virus, had the same annual transmission probability as untreated HIV+ temporary residents.
Model scenarios
To assess the impact of expanding ART access to all HIV+ temporary residents, we considered two modelling scenarios. First, we ran a ‘status quo’ scenario, where the proportion of HIV+ temporary residents virally suppressed remained constant and equalled the proportion at the start of ATRAS (58–68% on ART with 65–75% suppressed). We then ran an ‘expanded access’ scenario, where the proportion of the population taking ART and with suppressed virus changed over time to reach 90–100% of HIV+ temporary residents by 24 months, matching ATRAS data.14,15 For both scenarios, the annual probability an untreated HIV+ person transmits HIV to another person (β) retained the same value. This means the sexual behaviour of temporary residents and the effects of current prevention strategies are maintained for both scenarios. Any change in new infections is then due to the change in treatment coverage and the level of viral suppression.
Costs associated with ART provision
At enrolment, nearly all ATRAS patients already on ART were taking ART drug combinations recommended in current ART treatment guidelines.23 We assumed all patients were on and continued to take recommended ART drug combinations over the analysis period and assumed a range in the annual cost of providing ART (including the cost of medical care) of A $10000-A$20 000 based on previous work,12 as described in Table 1.
We estimated the ‘lifetime’ cost of providing care and treatment to sexual partners of temporary residents who become infected; this was based on the number of years a patient takes first, second, third and higher classes of drugs if they begin ART in their twenties.19 Using the cost estimates for each drug class in Australia12 with an assumed uncertainty of ±25% and adding annual medical care costs, gives a range in the undiscounted lifetime cost of providing ART post infection of $761 800-$1 269700 (Table 1). We applied a discount rate of 5% to all treatment costs. For discounting purposes, we assumed a conservative time between infection and initiating ART of 4–5 years.16 Note that this time between becoming infected with HIV and initiating ART reflects the estimated time to reach the average CD4 count at which HIV+ temporary residents in ATRAS started treatment, and does not include the effect of changing guidelines for initiating ART (see footnote 7, Table 1). To assess the sensitivity to this assumption, we also ran our analysis with a 2- to 4-year range in time between infection and initiating ART. This change produced only a minor increase (<10%) to our discounted cost estimates presented here, and did not affect the conclusions (the results of this analysis are available in the online repository).20
Results
During the first year after providing ART to all HIV+ temporary residents, there were 22 (interquartile range (IQR) :18–26) new infections caused by HIV+ temporary residents (Fig. 1). Expanding ATRAS to all HIV+ temporary residents and achieving almost universal viral suppression reduced annual new infections to a median of 5 (IQR: 3–7) after 5 years (Table 2; Fig. 1). Providing treatment and care to all HIV+ temporary residents averted a median of 80 new infections (IQR: 68.5–91.5) over 5 years (Table 2; Fig. 2a), at a median undiscounted cost of A$4 0820 000 (IQR: A$34 110 000–A $47 530 000) and a median discounted cost of A$36 240 000 (IQR: A$30 280 000–A$42 200 000) (taking the median of the sum for each simulation). This corresponds to a cost per infection averted of A$450 000 (IQR: A$360 000–A$540 000) (with 5% discounting).
Fig. 1.
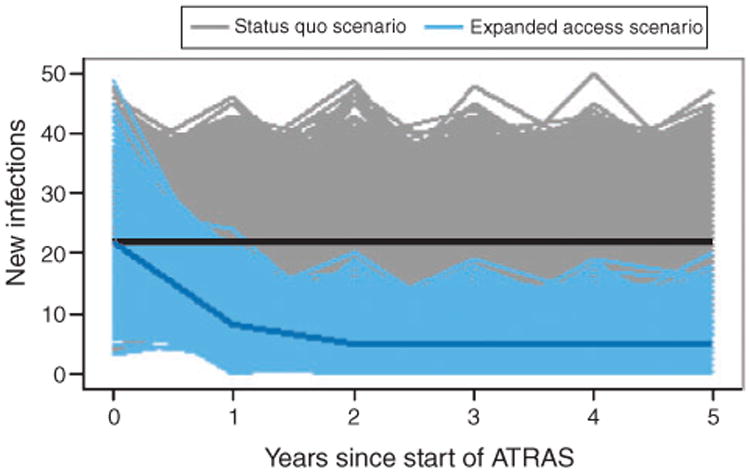
Annual number of new infections caused by HIV-positive (HIV+) temporary residents for each simulation. The grey lines represent the status quo scenario simulations, while the blue lines are for the expanded access scenario simulations (expansion of medical care and antiretroviral treatment (ART) to all temporary residents). The black and dark blue lines show the median number of new infections for the status quo and expanded access simulations respectively.
Table 2. Summary results for status quo scenario and the expanded access scenario.
Results show the median and interquartile range (IQR) of all simulations for each scenario. ART, antiretroviral treatment; NA, not applicable. Costs are, rounded to the nearest A$10 000
| Indicator | Status quo scenario (median, IQR) | Expanded access scenario (median, IQR) |
|---|---|---|
| Annual infections after 5 years | 22 (18–26) | 5 (3–7) |
| Cumulative infections | 131 (116–145) | 50 (42–57) |
| Infections averted | NA | 80 (68–91) |
| Cost of providing care and ART (undiscounted) | NA | A$40 820 000 (A$34 110 000-A$47 530 000) |
| Lifetime care and ART costs (undiscounted) | A$131 620 000 (A$111 230 000–A$152020 000) | A$50 570 000 (A$41450 000-A$59 700 000) |
| Reduction in lifetime care and ART costs (undiscounted) | NA | A$80 570 000(A$65 840 000-A$95 310 000) |
| Cost of providing care and ART (discounted 5%) | NA | A$36 240 000(A$30 280 000-A$42 200 000) |
| Lifetime care and ART costs (discounted 5%) | A$36640000 ($A31 490 000–A$41780000) | A$14 720 000 (A$12 300 000-A$17 140 000) |
| Reduction in lifetime care and ART costs (discounted 5%) | NA | A$21 730 000 (A$17 930 000-A$25 530 000) |
Fig. 2.
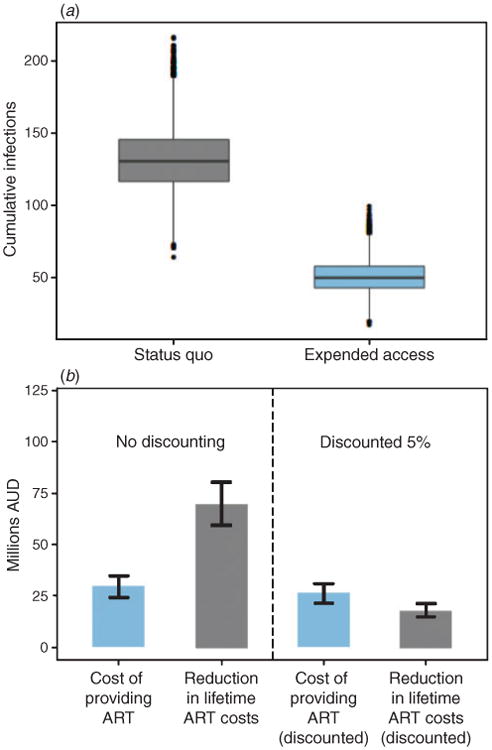
(a) Total number of new infections over 5 years for the expanded access scenario, where all HIV-positive (HIV+) temporary residents receive medical care and antiretroviral treatment (ART), compared with the status quo scenario, where ART coverage remains at current levels. (b) Median total costs for providing all HIV+ temporary residents with medical care and ART, and the reduction in lifetime treatment and care costs for partners of HIV+ temporary residents who acquire infection over 5 years. The bars show the interquartile range in total costs across all simulations.
Figure 2b shows the cumulative costs for providing ART and subsidised care to this population for the next 5 years compared with the savings due to the reduction in infections during this period. The resulting reduction in infections gives a median saving of A$80 570 000 (IQR: A$6 5840 000–A$95 310 000); Fig. 2b) in the lifetime treatment and care costs for newly infected people. When discounting is considered, the resulting savings in treatment and care costs reduces substantially (as lifetime costs are considered for infected partners) to a median of A$21730000 (IQR: A$17930 000–A$25530000).
Discussion
Our analysis showed that providing ART to HIV+ temporary residents within Australia will substantially reduce new infections and is unlikely to place a significant financial burden on the Australian Government — providing strong justifications for the provision of ART to temporary Australian residents. In our view, the only sustainable way to support optimal antiretroviral treatment for HIV+ temporary residents and achieve the resulting patient and population benefits over the long-term is through government subsidised ART. At the cessation of ATRAS in November 2015, 66 ATRAS patients and 400–500 HIV+ temporary residents nationally required an alternative mechanism for accessing ART. Pharmaceutical companies are currently providing access to antiretroviral treatment for Medicare ineligible patients on a compassionate basis in Australia. This is neither ideal nor sustainable. Applying for compassionate access is inefficient, time consuming for clinical staff, and delays treatment initiation. Numerous administrative barriers for specific treatments exist and not all antiretroviral drugs are available through compassionate access; this results in the use of suboptimal regimens instead of drugs, which are clinically indicated and medically appropriate. Furthermore, patients are often required to initially attempt sourcing ART from overseas, which leads to stress and further delays to achieving viral suppression. Overseas supplies of drugs are also often less safe and of lower quality. Finally, basing this important public health measure on the goodwill of commercial companies is subject to change, especially as generic antiretrovirals enter the Australian market from 2017. While the majority of HIV+ temporary residents within Australia eventually become permanent residents and eligible for ART under Medicare,15 results from the START trial24 and ATRAS14 show that any barriers and delays in treatment are detrimental to their health and are contrary to public health considerations.
Australia's current National HIV Strategy aims to have 90% of all HIV+ people on ART and to virtually eliminate transmission by 2020.25 To achieve ambitious HIV and ART targets across Australia will require a maximum focus on vulnerable and marginalised populations like HIV+ temporary residents, and must ensure they have access to quality information, support, testing and ART.
Several analysis limitations affect the results. Most importantly, due to data for sexual and injecting behaviour, sexual partner characteristics and prevention practices being unavailable, we were unable to develop a more detailed model of HIV transmission among temporary residents in Australia. The only data source providing data for HIV+ temporary residents is the ATRAS study, which is a clinical cohort with no associated behavioural- or transmission-related data. This meant our calculations required several simplifying assumptions to estimate the risk of transmission. We assumed transmission parameters for treated and untreated patients are constant over time, whereas there is evidence the risk of transmission has been decreasing over the last 10 years.22 We also assumed, due to limited data for temporary residents, HIV transmission from untreated HIV+ temporary residents is the same as for the overall Australian population of people living with HIV. Temporary resident populations are often culturally and linguistically diverse, with less engagement with care and lower knowledge of HIV and prevention methods — suggesting they may have a higher risk of transmission to their partners.26–28 Due to a lack of data on the partners of temporary residents, we were unable to consider onward transmission from newly infected partners of temporary residents. These assumptions may result in an underestimate for the number of new infections prevented. Counteracting this, however, is the assumption those with unsuppressed virus have the same transmission risk as those not taking ART, whereas treatment is likely to still have some efficacy even if it does not completely suppress a patient's viral load. In addition, the rate of partner change is constant due to an implicit homogenous mixing assumption, whereas many temporary residents could be in monogamous partnerships (and hence can only transmit HIV once) or have no sexual partners in Australia. Overestimating the number of new infections increases the cost-effectiveness of providing ART to the temporary resident population, while underestimating new infections decreases it. Finally, we also assumed partners of HIV+ temporary residents are Medicare eligible. This assumption likely lowers the cost of providing ART to HIV+ temporary residents, as some of their sexual partners may also be ineligible for Medicare.
By denying ART access to HIV+ temporary residents via Medicare, the Australian Government increases the risk of HIV transmission and the future long-term costs of providing HIV care and treatment, eroding any savings gained by denying HIV+ temporary residents access to subsidised treatment. The Australian Government already allows HIV+ temporary residents to live and work in Australia, and contribute economically through taxation. Providing these individuals with subsidised ART will cost little overall and help Australia achieve its National HIV Strategy targets.
Acknowledgments
Richard Gray designed the model, conducted the analysis, and was the lead writer. Kathy Petoumenos and Matthew Law had the initial idea for this analysis and contributed to the model design and analysis plan. Kathy Petoumenos, Jo Watson, Aaron Cogle, Don Smith, Jennifer Hoy, Robert Finlayson, Fraser Drummond, Bill Whittaker, Matthew Law contributed to the ATRAS study on which the analysis was based and assisted with procurement of data. All authors contributed to data interpretation, writing of the manuscript, and approved the submitted manuscript.
This analysis was conducted with support from the Australian Government Department of Health and Ageing and the Australian National Health and Medical Research Council (1050874).
The Australian HIV Observational Database is funded as part of the Asia Pacific HIV Observational Database, a program of The Foundation for AIDS Research, amfAR, and is supported in part by grant No. U01-AI069907 from the USA National Institutes of Health (with funding provided by the National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health & Human Development and the National Cancer Institute) and by unconditional grants from Boehringer-Ingelheim Pty Ltd., Bristol-Myers Squibb Australia Pty Ltd., Gilead Sciences Pty Ltd., GlaxoSmithKline, Janssen-Cilag Pty Ltd., Merck Sharp & Dohme (Australia) Pty Ltd., Roche Pty Ltd. and Pfizer Pty Ltd. The Kirby Institute for is funded by the Australian Government Department of Health, and is affiliated with the Faculty of Medicine, UNSW Sydney. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above.
AbbVie Pty Ltd, Boehringer Ingelheim Pty Ltd, Bristol-Myers Squibb Pty Ltd., Gilead Sciences Pty Ltd., Janssen-Cilag Pty Ltd., Merck Sharp & Dohme (Australia) Pty Ltd. and ViiV Healthcare Pty Ltd. provide ART on a compassionate basis to ATRAS patients via participating sites.
Both the National Association of People with HIV Australia (NAPWHA) and the ATRAS study partners acknowledge the people living with HIV organisations and networks across Australia supporting this work.
Footnotes
Conflicts of interest: Bill Whittaker, Jo Watson, Lisa Bastian have no conflicts of interest. Kathy Petoumenos and Matthew Law's institution have received an unrestricted research grant from Gilead Science. Kathy Petoumenos has conducted consultancy work for ViiV Healthcare. Richard Gray's institution has received funding for his research from The World Bank Group, UNAIDS and NSW Department of Health. Jennifer Hoy's institution has received funding for her participation on Advisory Boards for Merck Sharp & Dohme (Australia) Pty Ltd., Gilead Sciences Pty Ltd., ViiV Healthcare Pty Ltd. and Janssen-Cilag Pty Ltd. Don Smith has received research grants from Gilead Sciences Pty Ltd., ViiV Healthcare Pty Ltd., Merck Sharp & Dohme (Australia) Pty Ltd., Abbott Australasia Pty Ltd., Kirby institute, NSW Department of Health, and educational support from Gilead Sciences Pty Ltd., ViiV Healthcare Pty Ltd., Merck Sharp & Dohme (Australia) Pty Ltd., Abbott Australasia, Bristol-Myers Squibband the NSW Department of Health. Robert Finlayson has received consultancy fees and educational grants from Abbott Australasia Pty Ltd.t, Bristol-Myers Squibb Pty Ltd., Boerhinger Ingelheim Pty Ltd., Janssen-Cilag Pty Ltd., Gilead Sciences Pty Ltd., Merck Sharp & Dohme (Australia) Pty Ltd. and ViiV Pty Ltd. Fraser Drummond is currently employed by ViiV Healthcare Pty Ltd.
References
- 1.Bor J, Herbst AJ, Newell ML, Barnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science. 2013;339:961–5. doi: 10.1126/science.1230413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinkhof MWG, Boulle A, Weigel R, Messou E, Mathers C, Orrell C, Dabis F, Pascoe M, Egger M. International Epidemiological Databases to Evaluate AIDS (IeDEA) Mortality of HIV-infected patients starting antiretroviral therapy in sub-Saharan Africa: comparison with HIV-unrelated mortality. PLoS Med. 2009;6:e1000066. doi: 10.1371/journal.pmed.1000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egger M, May M, Ge C, Phillips AN, Ledergerber B, Dabis F, Costagliola D, D'Arminio Monforte A, de Wolf F, Reiss P, Lundgren JD, Justice AC, Staszewski S, Leport C, Hogg RS, Sabin CA, Gill MJ, Salzberger B, Sterne JA. ART Cohort Collaboration Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–29. doi: 10.1016/S0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 4.May M, Boulle A, Phiri S, Messou E, Myer L, Wood R, Keiser O, Sterne JA, Dabis F, Egger M. IeDEA Southern Africa and West Africa. Prognosis of patients with HIV-1 infection starting antiretroviral therapy in sub-Saharan Africa: a collaborative analysis of scale-up programmes. Lancet. 2010;376:449–57. doi: 10.1016/S0140-6736(10)60666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yiannoutsos CT, Johnson LF, Boulle A, Musick BS, Gsponer T, Balestre E, Law M, Shepherd BE, Egger M International Epidemiologic Databases to Evaluate AIDS (IeDEA) Collaboration. Estimated mortality of adult HIV-infected patients starting treatment with combination antiretroviral therapy. Sex Transm Infect. 2012;88:i33–43. doi: 10.1136/sextrans-2012-050658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim, Kumwenda J, Grinsztejn B, Pilotto JHS, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnell D, Baeten JM, Kiarie J, Thomas KK, Stevens W, Cohen CR, McIntyre J, Lingappa JR, Celum C. Partners in Prevention HSV/HIV Transmission Study Team Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–8. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eaton JW, Johnson LF, Salomon JA, Bärnighausen T, Bendavid E, Bershteyn A, Bloom DE, Cambiano V, Fraser C, Hontelez JA, Humair S, Klein DJ, Long EF, Phillips AN, Pretorius C, Stover J, Wenger EA, Williams BG, Hallett TB. HIV treatment as prevention: systematic comparison of mathematical models of the potential impact of antiretroviral therapy on HIV incidence in South Africa. PLoS Med. 2012;9:e1001245. doi: 10.1371/journal.pmed.1001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. World Health Organization; Geneva Switzerland: 2015. [PubMed] [Google Scholar]
- 11.Australian Government, Department of Health and Aging. The Pharmaceutical Benefits Scheme: Section 100 – Highly Specialised Drugs Program. Available online at: http://www.pbs.gov.au/info/browse/section-100/s100-highly-specialised-drugs [verified 14 July]
- 12.Schneider K, Gray RT, Wilson DP. A cost-effectiveness analysis of HIV pre-exposure prophylaxis for men who have sex with men in Australia. Clin Infect Dis. 2014;58:1027–34. doi: 10.1093/cid/cit946. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann S, Wardrop J, John M, Gaudieri S, Lucas M, Mallal S, Nolan D. The impact of visa status and Medicare eligibility on people diagnosed with HIV in Western Australia: a qualitative report. Sex Health. 2012;9:407–13. doi: 10.1071/SH11181. [DOI] [PubMed] [Google Scholar]
- 14.Petoumenos K, Watson J, Whittaker B, Hoy J, Smith D, Bastian L, Finlayson R, Sloane A, Wright ST, McManus H, Law MG. Subsidized optimal ART for HIV-positive temporary residents of Australia improves virological outcomes: results from the Australian HIV Observational Database Temporary Residents Access Study. J Int AIDS Soc. 2015;18:19392. doi: 10.7448/IAS.18.1.19392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Kirby Institute. The Australian HIV Observational Database Temporary Residents Access Study (ATRAS) One year follow-up. Sydney, NSW: The Kirby Institute, UNSW Australia; 2013. [Google Scholar]
- 16.Jansson J, Kerr CC, Mallitt KA, Wu J, Gray RT, Wilson DP. Inferring HIV incidence from case surveillance with CD4+ cell counts. AIDS. 2015;29:1517–25. doi: 10.1097/QAD.0000000000000679. [DOI] [PubMed] [Google Scholar]
- 17.The Kirby Institute. HIV in Australia Annual Surveillance Report Supplement. Sydney, NSW: The Kirby Institute, UNSW Australia; 2014. [Google Scholar]
- 18.Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, Corbelli GM, Estrada V, Geretti AM, Beloukas A, Asboe D, Viciana P, Gutiérrez F, Clotet B, Pradier C, Gerstoft J, Weber R, Westling K, Wandeler G, Prins JM, Rieger A, Stoeckle M, Kümmerle T, Bini T, Ammassari A, Gilson R, Krznaric I, Ristola M, Zangerle R, Handberg P, Antela A, Allan A, Phillips AN, Lundgren J. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA. 2016;316(2):171–81. doi: 10.1001/jama.2016.5148. [DOI] [PubMed] [Google Scholar]
- 19.Jansson J, Wilson DP, Carr A, Petoumenos K, Boyd MA. Currently available medications in resource-rich settings may not be sufficient for lifelong treatment of HIV. AIDS. 2013;27:1245–51. doi: 10.1097/QAD.0b013e32835e163d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray RT. Geneva: Zenodo; 2017. ART_For_Temporary_Residents: version corresponding to accepted paper [Data set] Available online at: https://zenodo.org/record/825366#.WX65LoSGPRY and https://github.com/leftygray/ART_For_Temporary_Residents. [DOI] [Google Scholar]
- 21.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2016. Available online at: https://www.R-project.org/ [verified 14 July 2017] [Google Scholar]
- 22.The Kirby Institute. Annual Surveillance Report. Sydney, NSW: The Kirby Institute, UNSW Australia; 2015. HIV, viral hepatitis and sexually transmissible infections in Australia. [Google Scholar]
- 23.Department of Health and Human Services. Panel on Antiretroviral Guidelines for Adults and Adolescents: Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available online at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf [verified 4 April 2016]
- 24.INSIGHT START Study Group. Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, Avihingsanon A, Cooper DA, Fätkenheuer G, Llibre JM, Molina JM, Munderi P, Schechter M, Wood R, Klingman KL, Collins S, Lane HC, Phillips AN, Neaton JD. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;2015:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Australia Government, Department of Health. Seventh National HIV Strategy 2014–2017. Available from: http://www.health.gov.au/internet/main/publishing.nsf/Content/ohp-bbvs-hiv [verified 7 July 2017]
- 26.Lemoh C, Guy R, Yohannes K, Lewis J, Street A, Biggs B, Hellard M. Delayed diagnosis of HIV infection in Victoria 1994 to 2006. Sex Health. 2009;6:117–22. doi: 10.1071/SH08028. [DOI] [PubMed] [Google Scholar]
- 27.Lemoh CN, Baho S, Grierson J, Hellard M, Street A, Biggs BA. African Australians living with HIV: a case series from Victoria. Sex Health. 2010;7:142–8. doi: 10.1071/SH09120. [DOI] [PubMed] [Google Scholar]
- 28.McMahon T, Moreton RJ, Luisi BN. Guarding against emerging epidemics: addressing HIV and AIDS among culturally and linguistically diverse communities in NSW. NSW Public Health Bull. 2010;21(4):83–5. doi: 10.1071/NB10012. [DOI] [PubMed] [Google Scholar]


