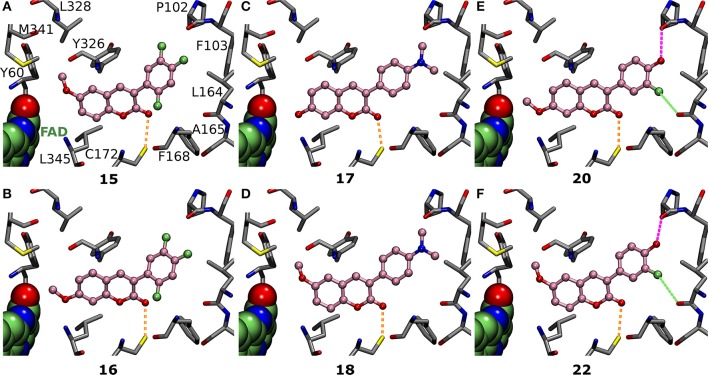
Figure 6.
The effects of R1 and R2 substituents are dependent on the 3-phenyl ring substituents. (A) Derivatives 15 (Figure 2; IC50 of 292 nM; Table 1) and (B) 16 (Figure 2; IC50 of 1433 nM; Table 1) both have fluorine groups at R4, R6, and R7 positions, but switching the coumarin ring's R1-methoxy into the R2 position reduces the inhibition by whopping 1141 nM. In contrast, with (C) 17 (Figure 2; IC50 of 384 nM; Table 1) and (D) 18 (Figure 2; IC50 of 617 nM; Table 1), the R1-methoxy does not elicit as strong inhibition as the R2-hydroxyl due to the overall coumarin ring alignment dictated by the 3-phenyl's R5-dimethylamine. The R1/R2-methoxy switch produces a completely opposite effect for (E) 20 (Figure 2; IC50 of 391 nM; Table 1) and (F) 22 (Figure 2; IC50 of 831 nM; Table 1) than it did for 15 and 16 (panels A,B); namely, it lowered the inhibition by 440 nM (Table 1). For further details, see Figures 1, 4.