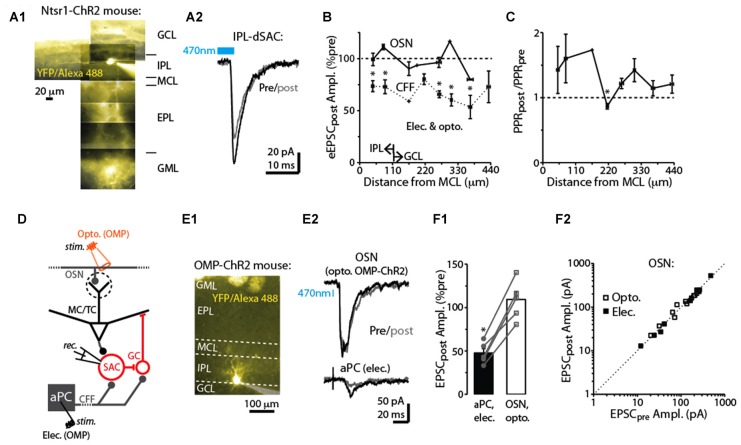
Figure 4.
Specificity of DSE in dSACs. (A1) Reconstructed epi-fluorescence image of an internal plexiform layer (IPL)-dSAC filled with Alexa 488 in a bulb slice from an ntsr1-ChR2-YFP mouse. Note dendrites restricted to the IPL and processes crossing through the external plexiform layer (EPL). Signals in the glomerular layer (GML) and GCL likely in part reflect ChR2-YFP. MCL = mitral cell layer. (A2) Mean light-evoked CFF-EPSCs (averages of 10 trials) recorded from the IPL-dSAC in (A1), both before (black) and 2.5 s (gray) after a 5 s depolarization to the test cell. (B) Summary of the effect of a 5-s depolarization of the test dSAC on the amplitude of EPSCs plotted vs. the distance between the dSAC cell body and the MCL. Note that for the CFF-EPSCs (n = 61 dSACs), the magnitude of DSE is independent of the location of the dSAC. Also plotted is a similar analysis performed on EPSCs evoked by stimulation of olfactory sensory neurons, OSNs (n = 21 dSACs; see example in Part E). Bins: 55 μm. The IPL/GCL border was located approximately 110 μm from the MCL (arrows). *p < 0.05, n = 7–14. (C) Summary of the effect of dSAC depolarization on the PPR of CFF-EPSCs vs. distance between the dSAC cell body and the MCL. Same experiments as CFF data in Part (B). *p = 0.022, paired t-test, n = 7. (D) Protocol for comparing CCF-EPSCs in dSACs with feedforward EPSCs evoked by stimulation of OSN inputs. Feedforward EPSCs were evoked using light pulses in olfactory marker protein (OMP)-ChR2-YFP mice (as shown) or with electrical stimulation of OSNs. (E) Feedforward EPSCs evoked in a dSAC were not impacted by dSAC depolarization. Illustrated is the image of the Alexa Fluor-filled IPL-dSAC (E1) and current traces in the same cell evoked by optogenetic OSN stimulation (E2, top) and electrical stimulation in aPC (E2, bottom). In the image in (E1), note the fluorescent glomeruli at top reflecting ChR2-YFP. (F1) In five dSACs in which both OSN-driven excitatory currents and CFF-EPSCs were recorded, depolarization of the test cell reduced the CFF-EPSC without impacting the OSN-driven current. Five to 20 trials were recorded in each cell for each type of stimulus. *p < 0.001, paired t-test, n = 5. (F2) Effect of dSAC depolarization on OSN-driven excitatory currents in 20 dSACs. Experiments were separated by whether currents were light evoked (in OMP-ChR2 mice; open symbols) or by electrical stimulation in the OSN layer (filled symbols).