Figure 7.
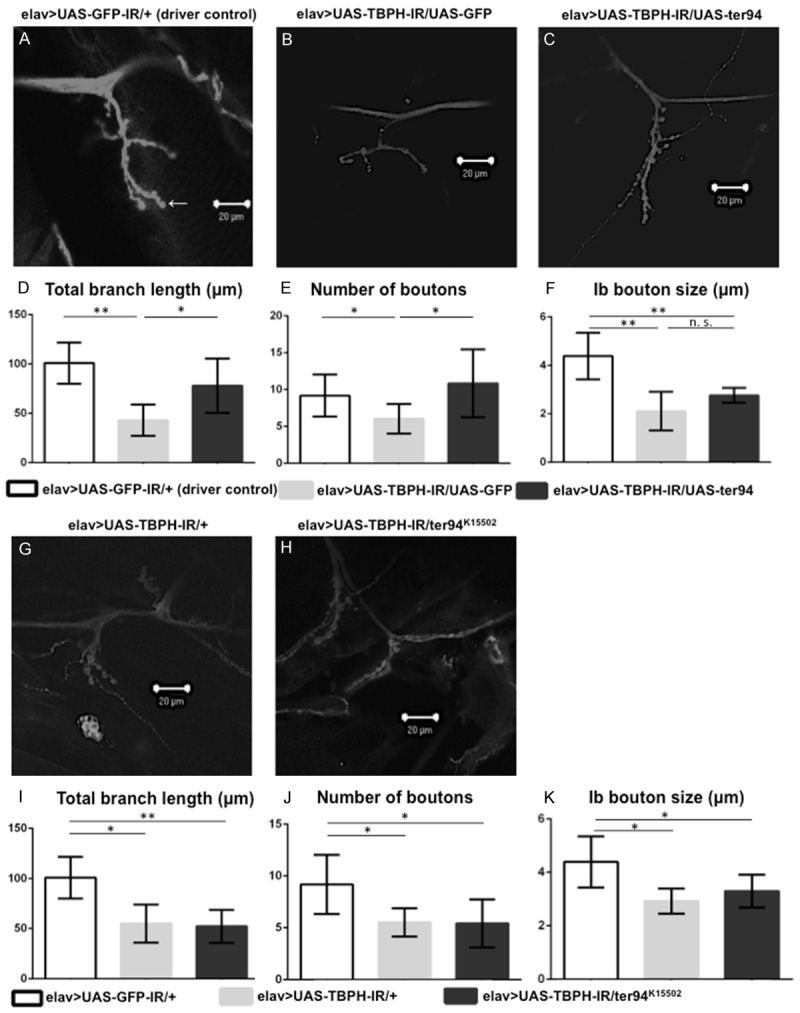
Overexpression of wild-type ter94 improves the morphology of NMJs in TBPH knockdown larvae. A loss-of-function ter94 mutation did not change the morphology of the MN presynaptic terminals at NMJs in neuron-specific TBPH knockdown larvae. A representative image of anti-HRP staining of muscle 4 synapses in third instar larvae carrying yw/Y; UAS-GFP-IR/+; elav-GAL4/+ (elav>UAS-GFP-IR/+, A; a driver control), yw/Y; UAS-TBPH-IR517-531/UAS-GFP; elav-GAL4/+ (elav>UAS-TBPH-IR/UAS-GFP, B; a responder control), yw/Y; UAS-TBPH-IR517-531/UAS-ter94; elav-GAL4/+ (elav>UAS-TBPH-IR/UAS-ter94, C), yw/Y; UAS-TBPH-IR517-531/+; elav-GAL4/+ (elav>UAS-TBPH-IR/+, G), and yw/Y; UAS-TBPH-IR517-531/ter94K15502+; elav-GAL4/+ (elav>UAS-TBPH-IR/ter94K15502, H). (D and I) Total branch length of the NMJ from muscle 4 for each of the indicated genotypes is shown. Compared to the total length of synaptic branches of MNs in driver control larvae (A), the length in larvae carrying elav>UAS-TBPH-IR/UAS-GFP (B) was significantly decreased (P < 0.001, n = 6, D). Conversely, the total branch length of MNs in larvae with ter94 overexpression on the background of neuron-specific TBPH knockdown (C) was significantly longer than that in larvae carrying elav>UAS-TBPH-IR/UAS-GFP (B) (P < 0.05, n = 6, D). The total branch length in neuron-specific TBPH knockdown larvae (G) was significantly decreased (P < 0.001, n = 6, I). This decrease in branch length observed in the neuron-specific TBPH knockdown larvae (G) was almost the same as in larvae carrying the neuron-specific TBPH knockdown crossed with the loss-of-function allele of ter94 (H) (P = 0.7, n = 7, I). (E and J) The number of synaptic boutons for each of the indicated genotypes is shown. The number of synaptic boutons in larvae carrying wild-type ter94 overexpression on the background of neuron-specific TBPH knockdown (C) was significantly increased compared to larvae carrying elav>UAS-TBPH-IR/UAS-GFP (B) (P < 0.05, n = 6, E). Compared to larvae carrying elav>UAS-GFP-IR/+, the number of synaptic boutons is significantly decreased in larvae carrying elav>UAS-TBPH-IR/UAS-GFP (P < 0.001, n = 6, E). The number of synaptic boutons in larvae carrying elav>UAS-TBPH-IR/UAS-ter94 is almost the same as that in driver control larvae. The number of synaptic boutons of MNs in neuron-specific TBPH knockdown larvae (G) was also significantly decreased compared to driver control larvae (A) (P < 0.05, n = 6, J). This decrease in the number of synaptic boutons in neuron-specific TBPH knockdown larvae was almost the same in larvae carrying the neuron-specific TBPH knockdown crossed with the loss-of-function allele of ter94 (H) (P = 0.9, n = 7, J). (F and K) The size of synaptic boutons for each of the indicated genotypes is shown. The size of synaptic Ib boutons (indicated with an arrow in A; the Ib boutons measured in this paper are the largest boutons in the NMJs) was measured (n = 6 for elav>UAS-GFP-IR, n = 6 for elav>UAS-TBPH-IR/UAS-GFP, n = 6 for elav>UAS-TBPH-IR/UAS-ter94, n = 6 for elav>UAS-TBPH-IR, and n = 7 for elav>UAS-TBPH-IR/ter94K15502). We found no significant differences in the size of synaptic boutons between larvae with elav>UAS-TBPH-IR/UAS-GFP (B) and elav>UAS-TBPH-IR/UAS-ter94 (C), or between those with elav>UAS-TBPH-IR (G) and elav>UAS-TBPH-IR/ter94K15502 (H). The size was significantly smaller in neuron-specific TBPH knockdown larvae (2.9 ± 0.2 µm, G) than in control larvae (4.3 ± 0.4 µm, A) (P < 0.01, K). Columns and horizontal bars show the mean and SE of the measurements, respectively. **P < 0.01, and *P < 0.05. The scale bars indicate 20 µm (A, B, C, G, and H).
