Abstract
The aim of this meta-analysis is to evaluate the analgesic effects of intra-articular dexmedetomidine (DEX) in arthroscopic knee surgery. A comprehensive literature search was conducted to identify randomized controlled trials (RCTs) comparing intra-articular DEX versus control for postoperative analgesia in knee arthroscopy. Trial sequential analysis (TSA) was applied to determine the reliability of the evidence. Twelve RCTs including 594 patients met the eligibility criteria. DEX treatment significantly improved postoperative pain outcomes, with weighted mean differences (95% confidence interval) between the DEX and control groups of −1.57 (−1.94 to −1.20, P < 0.00001) for pain scores at rest at postoperative 1 h, −8.54 mg (−11.96 to −5.13, P < 0.00001) for morphine-equivalents at postoperative 0–24 h, and 257.57 min (209.86 to 305.28, P < 0.00001) for time to first request for postoperative analgesia. TSA indicated there is sufficient evidence for these outcomes. Intra-articular DEX did not affect the incidence of postoperative nausea and vomiting, hypotension, bradycardia, or somnolence. This meta-analysis demonstrated that intra-articular administration of DEX improved pain outcomes in the early postoperative period after knee arthroscopy. Due to the limited number of trials and patients included in this meta-analysis, more evidence is required to confirm these findings.
Introduction
Arthroscopic knee surgery is a common orthopedic surgical procedure1. Patients are managed on a day-case basis; therefore, adequate postoperative pain relief is critical to facilitate patient discharge and early rehabilitation. A simple method of analgesia with a prolonged duration of action and minimal adverse effects is required.
Systemic dexmedetomidine (DEX) produces sedative, analgesic, sympatholytic, and anesthetic-sparing effects2. Evidence suggests that intra-articular administration of local anesthetics, opioids, magnesium, clonidine, and DEX, alone or in combination, decreases postoperative pain without significant adverse effects3–7. A combination of a local anesthetic agent and DEX, applied at the end of surgery, which targets peripheral nociceptive receptors may be an ideal protocol for pain control after knee arthroscopy.
To the author’s knowledge, there are no published meta-analyses investigating the effects of intra-articular administration of DEX on postoperative pain after knee arthroscopic procedures. This study was designed to determine the benefits and adverse effects of intra-articular administration of DEX in arthroscopic knee surgery. Trial sequential analysis (TSA) was carried out to assess the reliability of the findings.
Materials and Methods
This systematic review and meta-analysis was performed in accordance with the PRISMA statement and the recommendations of the Cochrane Collaboration8,9 (Supplementary Table S1). The protocol is registered on PROSPERO (registration number: CRD42017059593).
Search strategy
Two review authors (KP, WRC) independently searched the PubMed, EMBASE, CENTRAL, CNKI, and WanFang databases from inception to March 15, 2017 using MeSH terms combined with text words, without restriction on language. A manual search of the reference lists from relevant articles was also carried out. The search strategies for PubMed, EMBASE, and CENTRAL are summarized in Supplementary Table S2.
Study selection
Two authors (KP, WRC) independently examined titles and abstracts and reviewed articles to select eligible studies. Inclusion criteria were: (1) study design: randomized controlled trial (RCT); (2) participants: adult patients undergoing arthroscopic knee surgery; (3) comparisons: intra-articular DEX versus saline control, or intra-articular DEX combined with a local anesthetic versus local anesthetic alone; and (4) outcomes: postoperative pain scores, opioid requirement, time to first request for postoperative analgesia, and incidence of postoperative nausea and vomiting (PONV), hypotension, bradycardia, or somnolence. Exclusion criteria were: (1) study design other than a RCT; (2) reviews, letters, abstracts, or editorials; or (3) studies that reported insufficient data. Disagreements about study selection were resolved by group discussion and consensus.
Data extraction
Two authors (KP, WRC) independently extracted data from each eligible RCTs, including first author, publication year, number of patients, type of anesthesia, type of intraoperative and postoperative analgesia, and outcome measures.
Primary outcome measures were postoperative pain and cumulative opioid consumption. Postoperative pain was measured as pain at rest at postoperative 1, 2, 4, 6, 8, 12, and 24 h and on movement at postoperative 1, 2, and 8 h expressed on a visual analog scale (VAS), where 0 = no pain and 10 = the most severe pain. Opioid consumption during postoperative 0–24 h was expressed in milligrams of morphine equivalent, where intravenous (i.v.) morphine 10 mg = i.v. tramadol 100 mg = i.v. fentanyl 0.1 mg10,11. Secondary outcomes were time to first request for postoperative analgesia and incidence of PONV, hypotension, bradycardia, or somnolence.
Disagreements about data extraction were resolved by group discussion and consensus.
Quality assessment
Two authors (KP, XWM) independently graded the methodological quality of each RCT and the quality of evidence for each outcome measure.
The methodological quality was assessed with the Cochrane risk of bias tool9. Seven domains (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias [baseline characteristics, funding]) were examined. Risk of bias was categorized as high (one or more domains were high risk), low (all domains were low risk), or unclear (other).
The quality of evidence for each outcome measure was evaluated with Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology (https://gradepro.org)12. Evidence was categorized as high, moderate, low, or very low according to five criteria: risk of bias, inconsistency, indirectness, imprecision, and publication bias.
Publication bias was assessed using Begg’s rank correlation test and Egger’s linear regression test13,14.
Disagreements about quality assessment were resolved by group discussion and consensus.
Statistical analysis
Statistical analyses were performed using STATA 14.0 (Stata Corp, College Station, TX).
To describe the effect size of an intervention, risk ratios (RRs) with 95% confidence intervals (CIs) were calculated for dichotomous data, and weighted mean differences (WMDs) with 95% CIs were calculated for continuous variables. A random-effects model was used in this meta-analysis15. Heterogeneity was measured and expressed as I2, with I2 > 50% indicating significant heterogeneity16. Subgroup analyses were conducted for the outcome measures, stratified by allocation concealment (adequate or unclear), use of local anesthetic (yes or no), type of postoperative analgesic (opioids or none opioids), use of postoperative patient-controlled analgesia (PCA, yes or no), and DEX dose (≤1 µg/kg or >1 µg/kg). Sensitivity analysis was performed by omitting one study at a time to assess the effect of a single comparison on the overall estimates17. P < 0.05 denoted statistical significance.
As Type I and Type II errors may arise in a meta-analysis due to small sample size18, we assessed the reliability of the current results using TSA (version 0.9.5.5 beta, http://www.ctu.dk/tsa)18,19. If the cumulative Z curve crosses the trial sequential monitoring boundary or the futility boundary in the TSA diagram, it is unlikely that an anticipated effect is absent, or that further studies will change the inference. The required information size was calculated using a Type I error of 5%, a power of 80%, and intervention effects in RCTs with adequate allocation concealment15,20.
Results
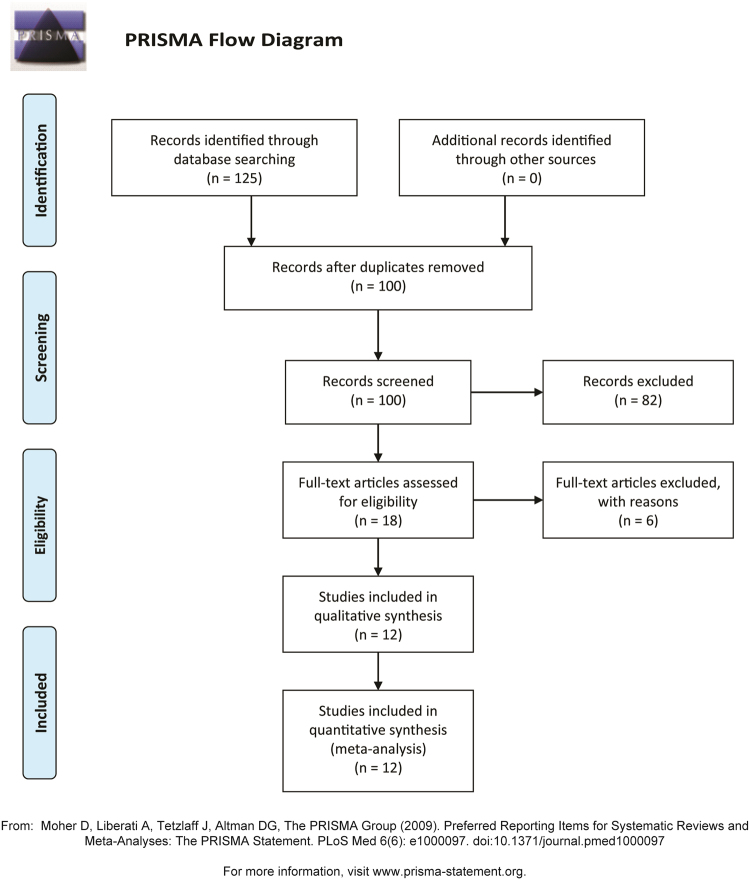
The initial literature search identified 125 articles. Of these, 113 were excluded because they failed to meet the eligibility criteria or were duplicates. A total of 12 RCTs involving 594 participants were finally included in this meta-analysis7,21–31. The PRISMA flow diagram is shown in Fig. 1.
Figure 1.
PRISMA flow diagram.
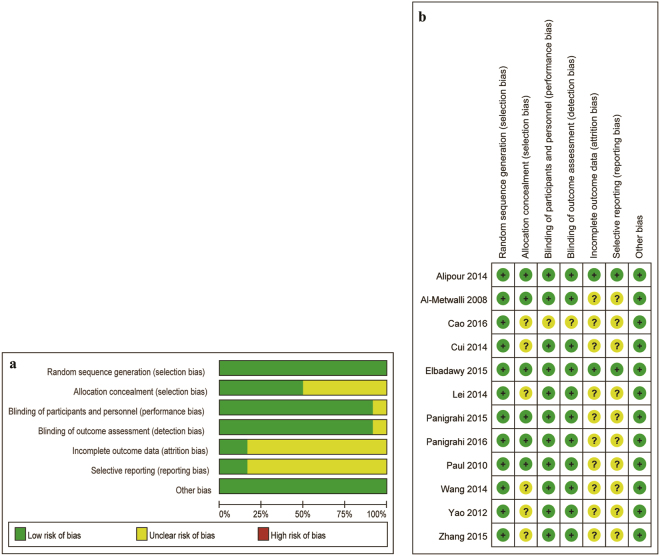
The characteristics of the included trials are provided in Table 1. Five trials compared intra-articular DEX with saline7,21,22,25,30, and seven trials compared a combination of DEX and a local anesthetic to local anesthetic alone23,24,26–29,31. The risk assessment is presented in Fig. 2. All trials were randomized and double-blind, with adequate allocation concealment in 6 trials7,21,24,26–28.
Table 1.
Study characteristics.
| Author, year | Country | Groups and interventions | (n) | Age (y), % males | Time of treatment | Anesthesia | Intra/postoperative analgesia | Main outcomes |
|---|---|---|---|---|---|---|---|---|
| Al-Metwalli, 2008 | Saudi Arabia | 1. DEX 1 μg/kg + saline to 20 ml 2. Saline 20 ml |
20 20 |
40.2, 55% 38.2, 65% |
Before tourniquet release | General | Fentanyl/diclofenac | Pain VAS at 1–24 h, diclofenac use 0–24 h, time to first analgesia, PONV, hypotension, and bradycardia |
| Alipour, 2014 | Iran | 1. DEX 1 µg/kg + saline to 25 ml 2. Saline 25 ml |
21 23 |
30.8, 71.4% 29.4, 73.9% |
Before tourniquet release | General | Fentanyl/tramadol | Pain VAS at 1–24 h, tramadol use 0–24 h, time to first analgesia, PONV, hypotension, bradycardia, and pruritus |
| Cao, 2016 | China | 1. DEX 100 µg + saline to 15 ml 2. DEX 50 µg + saline to 15 ml 3. Saline 15 ml |
20 20 20 |
42.1, 50% 44.1, 35% 42.2, 40% |
Before the end of surgery | General | Fentanyl/flurbiprofen | Pain VAS at 1–24 h, flurbiprofen use 0–24 h, time to first analgesia, PONV, hypotension, bradycardia, and somnolence |
| Cui, 2014 | China | 1. DEX 1 µg/kg + 0.25% bupivacaine to 20 ml 2. 0.25% bupivacaine 20 ml |
20 20 |
40.2, 40% 39.4, 4% |
Before the end of surgery | General | Fentanyl/PCA with fentanyl | Pain VAS at 1–24 h, fentanyl use 0–24 h, time to first analgesia, PONV, hypotension, bradycardia, and somnolence |
| Elbadawy, 2015 | Egypt | 1. DEX 1 µg/kg + 0.25% bupivacaine to 25 ml 2. 0.25% bupivacaine 25 ml |
25 25 |
36.5, 56% 33.6, 64% |
Before tourniquet release | General | Fentanyl/paracetamol | Pain VAS at 0.5–12 h, paracetamol use 0–24 h, time to first analgesia, PONV, hypotension, bradycardia, and hallucination |
| Lei, 2014 | China | 1. DEX 1 μg/kg + saline to 20 ml 2. Saline 20 ml |
20 20 |
40.0, 40% 39.0, 45% |
Before the end of surgery | General | Fentanyl/lornoxicam | Pain VAS at 1–24 h, lornoxicam use 0–24 h, time to first analgesia, PONV, hypotension, and bradycardia |
| Panigrahi, 2015 | India | 1. DEX 1 µg/kg + 0.2% ropivacaine to 20 ml 2. DEX 2 µg/kg + 0.2% ropivacaine to 20 ml 3. 0.2% ropivacaine 20 ml |
20 20 20 |
31.7, 75% 32.3, 65% 30.8, 70% |
Before tourniquet release | Spinal | Bupivacaine/diclofenac | Pain VAS at 1–24 h, diclofenac use 0–24 h, time to first analgesia, PONV, hypotension, and bradycardia |
| Panigrahi, 2016 | India | 1. DEX 1 µg/kg + 0.2% ropivacaine to 20 ml 2. 0.2% ropivacaine 20 ml |
20 20 |
31.7, 75% 30.8, 70% |
Before tourniquet release | Spinal | Bupivacaine/diclofenac | Pain VAS at 1–24 h, diclofenac use 0–24 h, time to first analgesia, PONV, hypotension, and bradycardia |
| Paul, 2010 | India | 1. DEX 100 µg + 0.25% ropivacaine to 20 ml 2. 0.25% ropivacaine 20 ml |
30 30 |
41.4, 70% 39.8, 73% |
Before arthroscope removal | General | Fentanyl/PCA with fentanyl | Pain VAS at 1–18 h, fentanyl use 0–24 h, time to first analgesia, PONV, hypotension, bradycardia, and somnolence |
| Wang, 2014 | China | 1. DEX 1 µg/kg + 0.25% ropivacaine to 20 ml 2. 0.25% ropivacaine 20 ml |
30 30 |
40.0, 46% 40.0, 56% |
Before tourniquet release | General | Fentanyl/fentanyl | Pain VAS at 1–24 h, fentanyl use 0–24 h, time to first analgesia, PONV, hypotension, bradycardia, and hypoxemia |
| Yao, 2012 | China | 1. DEX 0.7 μg/kg + saline to 15 ml 2. Saline 15 ml |
20 20 |
40.2, 55% 39.2, 65% |
Before tourniquet release | General | Fentanyl/tramadol | Pain VAS at 1–12 h, tramadol use 0–24 h, time to first analgesia, PONV, hypotension, and bradycardia |
| Zhang, 2015 | China | 1. DEX 100 µg + 0.25% ropivacaine to 20 ml 2. 0.25% ropivacaine 20 ml |
30 30 |
40.4, 63% 38.9, 66% |
Before the end of surgery | General | Fentanyl/PCA with fentanyl | Pain VAS at 1–24 h, fentanyl use 0–24 h, time to first analgesia, PONV, hypotension, bradycardia, and somnolence |
DEX, dexmedetomidine; VAS, visual analogue scale score; PONV, postoperative nausea and vomiting; PCA, patient controlled analgesia.
Figure 2.
Risk of bias assessment. (a) Risk of bias graph; (b) risk of bias summary.
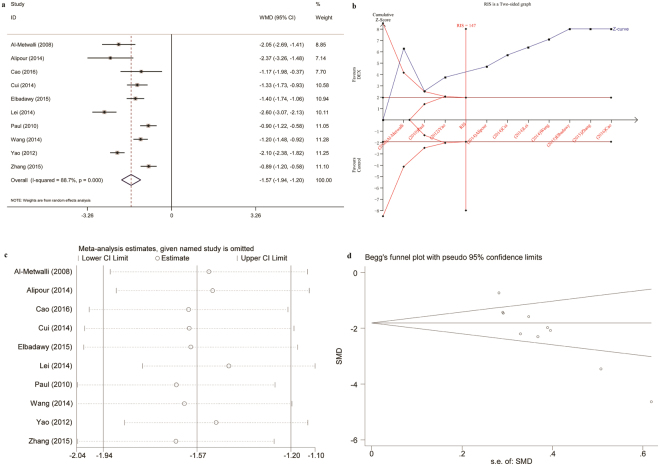
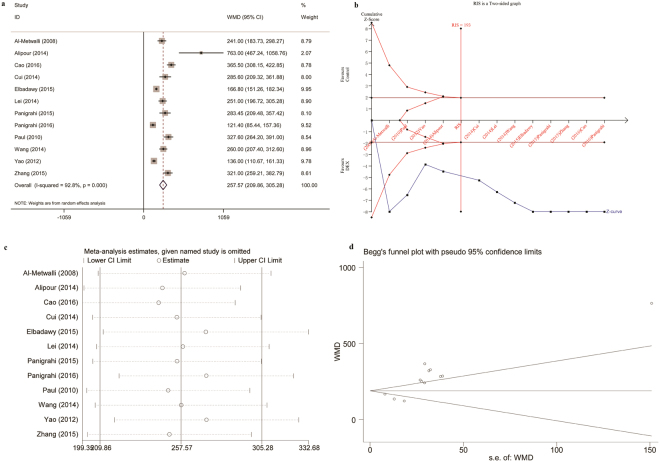
The primary and secondary outcomes are summarized in Table 2. The meta-analysis demonstrated significantly lower postoperative pain intensity at rest (1 h postoperatively: WMD, −1.57 [95% CI −1.94 to −1.20]; P = 0.0001) in patients treated with intra-articular DEX or a combination of DEX and local anesthetic compared to saline or local anesthetic alone (Fig. 3a). This statistically significant difference was also observed at postoperative 2 to 24 h and at 3 time points for pain intensity on movement. According to the TSA, the required information size of 147 patients was reached and the cumulative Z curve crossed the trial sequential monitoring boundary, indicating there is sufficient evidence for this outcome (Fig. 3b). Sensitivity analysis demonstrated these findings were robust (Fig. 3c), with pooled WMDs ranging from −1.65 (95% CI −2.04 to −1.26) to −1.44 (95% CI −1.78 to −1.10). Begg’s funnel plot (P = 0.002, Fig. 3d) and Egger’s test (P < 0.0001) indicated publication bias.
Table 2.
Outcomes.
| Outcomes | References | DEX (n) | Control (n) | Estimated benefit [95% CI] | P value | I2 (%) |
|---|---|---|---|---|---|---|
| Pain intensity at rest | ||||||
| 1 h postoperatively | 7,21–25,28–31 | 256 | 238 | WMD = −1.57 [−1.94, −1.20] | 0.00001 | 89 |
| 2 h postoperatively | 7,22–25,28–31 | 235 | 215 | WMD = −1.46 [−1.82, −1.10] | 0.00001 | 89 |
| 4 h postoperatively | 7,24,25,29–31 | 145 | 145 | WMD = −1.37 [−1.76, −0.97] | 0.00001 | 84 |
| 6 h postoperatively | 7,21,23–25,28,30,31 | 186 | 188 | WMD = −1.26 [−1.61, −0.92] | 0.00001 | 83 |
| 8 h postoperatively | 7,22,29–31 | 140 | 120 | WMD = −1.02 [−1.41, −0.64] | 0.00001 | 70 |
| 12 h postoperatively | 7,21,24,25,29–31 | 166 | 168 | WMD = −0.55 [−0.88, −0.23] | 0.0009 | 69 |
| 24 h postoperatively | 7,21–23,25,29,31 | 181 | 163 | WMD = −0.34 [−0.68, 0.00] | 0.05 | 59 |
| Pain intensity on movement | ||||||
| 1 h postoperatively | 22,29,30 | 90 | 70 | WMD = −1.71 [−2.39, −1.03] | 0.00001 | 86 |
| 2 h postoperatively | 22,29,30 | 90 | 70 | WMD = −1.80 [−2.25, −1.36] | 0.00001 | 70 |
| 8 h postoperatively | 22,29,30 | 90 | 70 | WMD = −1.29 [−1.57, −1.02] | 0.00001 | 32 |
| Other outcomes | ||||||
| Morphine-equivalents 0–24 h (mg) | 21,23,28–31 | 151 | 153 | WMD = −8.54 [−11.96, −5.13] | 0.00001 | 95 |
| Time to first analgesic request (min) | 7,21–31 | 316 | 278 | WMD = 257.57 [209.86, 305.28] | 0.00001 | 93 |
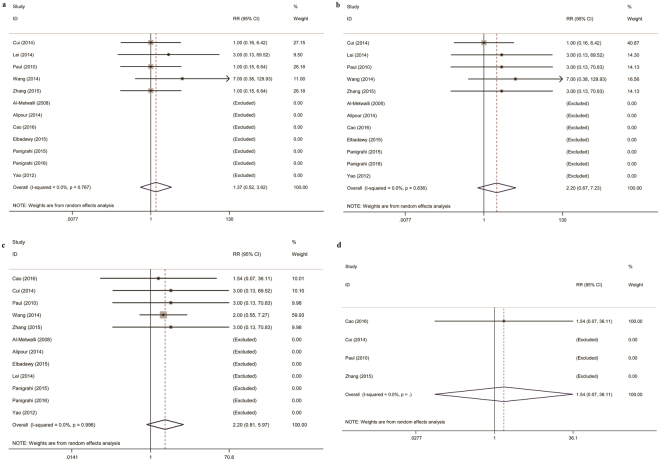
| PONV | 7,21–31 | 316 | 278 | RR = 1.37 [0.52, 3.62] | 0.52 | 0 |
| Hypotension | 7,21–31 | 316 | 278 | RR = 2.20 [0.67, 7.23] | 0.19 | 0 |
| Bradycardia | 7,21–31 | 316 | 278 | RR = 2.20 [0.81, 5.97] | 0.12 | 0 |
| Somnolence | 22,23,28,31 | 120 | 100 | RR = 1.54 [0.07, 36.11] | 0.79 | 0 |
DEX group vs. control group for all comparisons.
Pain intensity was scored with a visual analog scale as: 0 = no pain, 10 = the most severe pain imaginable.
Morphine-equivalents were calculated as: morphine 10 mg = tramadol 100 mg = fentanyl 0.1 mg, intravenously.
DEX, dexmedetomidine; PONV, postoperative nausea and vomiting; WMD, weighted mean difference; RR, risk ratio; CI, confidence interval.
Figure 3.
Intra-articular DEX versus control in knee arthroscopy: pain intensity at rest at 1 h postoperatively. (a) Forest plot; (b) Trial sequential analysis; (c) Sensitivity analysis; (d) Begg’s funnel plot. DEX, dexmedetomidine; WMD, weighted mean difference; RIS, required information size.
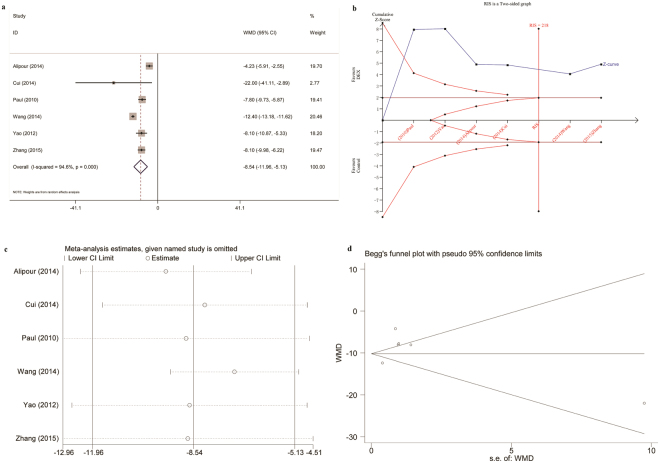
At postoperative 0–24 h, opioid consumption was significantly reduced with a WMD of −8.54 mg morphine-equivalents in patients treated with intra-articular DEX or a combination of DEX and local anesthetic compared to saline or local anesthetic alone (Fig. 4a,b). Sensitivity analysis demonstrated these findings were robust, with pooled WMDs ranging from −9.48 mg (95% CI −12.37 to −6.58) to −7.16 mg (95% CI −9.33 to −5.00) (Fig. 4c). No evidence of publication bias was observed on Begg’s funnel plot (P = 0.707, Fig. 4d) or Egger’s test (P = 0.328).
Figure 4.
Intra-articular DEX versus control in knee arthroscopy: morphine-equivalents 0–24 h postoperatively. (a) Forest plot; (b) Trial sequential analysis; (c) Sensitivity analysis; (d) Begg’s funnel plot. DEX, dexmedetomidine; WMD, weighted mean difference; RIS, required information size.
The use of intra-articular DEX or a combination of DEX and local anesthetic led to a longer time to first request for postoperative analgesia compared to saline or local anesthetic alone (WMD = 257.57 min) (Fig. 5a,b). Sensitivity analysis demonstrated these findings were robust, with pooled WMDs ranging from 244.42 min (95% CI 199.35 to 289.49) to 272.65 min (95% CI 218.34 to 326.96) (Fig. 5c). Begg’s funnel plot (P = 0.064, Fig. 5d) and Egger’s test (P = 0.002) indicated publication bias.
Figure 5.
Intra-articular DEX versus control in knee arthroscopy: time to first analgesic request. (a) Forest plot; (b) Trial sequential analysis; (c) Sensitivity analysis; (d) Begg’s funnel plot. DEX, dexmedetomidine; WMD, weighted mean difference; RIS, required information size.
For adverse effects, there were no significant differences in the incidence of PONV, hypotension, bradycardia, or somnolence between the DEX and control groups (Fig. 6a–d).
Figure 6.
Intra-articular DEX versus control in knee arthroscopy: adverse effects. (a) Postoperative nausea and vomiting; (b) Hypotension; (c) Bradycardia; (d) Somnolence. DEX, dexmedetomidine; WMD, weighted mean difference.
Subgroup analyses are shown in Table 3. Use of local anesthetic (yes or no) and postoperative PCA (yes or no) and DEX dose (≤1 µg/kg or >1 µg/kg) may account for the heterogeneity in some of the findings.
Table 3.
Subgroup analyses.
| Subgroups | No. of studies | Mean difference [95% CI] | P value | I 2 (%) | Between subgroup significance |
|---|---|---|---|---|---|
| Pain intensity at rest at 1 h postoperatively | |||||
| Total | 10 | −1.57 [−1.94, −1.20] | 0.00001 | 89 | |
| Allocation concealment | |||||
| Adequate | 4 | −1.58 [−2.15, −1.01] | 0.00001 | 83 | 0.95 |
| Unclear | 6 | −1.55 [−2.07, −1.04] | 0.00001 | 92 | |
| Local anesthetic use | |||||
| Yes | 5 | −1.13 [−1.34, −0.93] | 0.00001 | 50 | 0.0001 |
| No | 5 | −2.11 [−2.50, −1.73] | 0.00001 | 59 | |
| Postoperative analgesics | |||||
| Opioids | 6 | −1.41 [−1.86, −0.96] | 0.00001 | 90 | 0.31 |
| None opioids | 4 | −1.83 [−2.50, −1.15] | 0.00001 | 85 | |
| Postoperative PCA use | |||||
| Yes | 3 | −1.01 [−1.27, −0.76] | 0.00001 | 43 | 0.002 |
| No | 7 | −1.83 [−2.26, −1.39] | 0.00001 | 86 | |
| DEX dosage | |||||
| ≤1 µg/kg | 7 | −1.82 [−2.23, −1.40] | 0.00001 | 86 | 0.0001 |
| >1 µg/kg | 3 | −0.91 [−1.13, −0.70] | 0.00001 | 0 | |
| Morphine-equivalents 0– 2 4 h postoperatively (mg) | |||||
| Total | 6 | −8.54 [−11.96, −5.13] | 0.00001 | 95 | |
| Allocation concealment | |||||
| Adequate | 2 | −5.98 [−9.48, −2.48] | 0.0008 | 87 | 0.10 |
| Unclear | 4 | −10.02 [−13.37, −6.68] | 0.00001 | 88 | |
| Local anesthetics use | |||||
| Yes | 4 | −9.88 [−13.27, −6.49] | 0.00001 | 91 | 0.13 |
| No | 2 | −6.00 [−9.78, −2.22] | 0.002 | 82 | |
| Postoperative PCA use | |||||
| Yes | 3 | −8.03 [−9.44, −6.62] | 0.00001 | 5 | 0.94 |
| No | 3 | −8.28 [−14.06, −2.50] | 0.005 | 97 | |
| DEX dosage | |||||
| ≤1 µg/kg | 4 | −9.20 [−14.78, −3.62] | 0.001 | 96 | 0.67 |
| >1 µg/kg | 2 | −7.95 [−9.30, −6.61] | 0.00001 | 0 | |
| Time to first analgesic request (min) | |||||
| Total | 12 | 257.57 [209.86, 305.28] | 0.00001 | 93 | |
| Allocation concealment | |||||
| Adequate | 6 | 246.70 [177.68, 315.72] | 0.00001 | 92 | 0.70 |
| Unclear | 6 | 268.03 [185.08, 350.97] | 0.00001 | 94 | |
| Local anesthetics use | |||||
| Yes | 7 | 247.75 [187.67, 307.83] | 0.00001 | 92 | 0.49 |
| No | 5 | 291.85 [182.51, 401.20] | 0.00001 | 95 | |
| Postoperative analgesics | |||||
| Opioids | 6 | 298.74 [202.20, 395.27] | 0.00001 | 94 | 0.28 |
| None opioids | 6 | 234.38 [170.64, 298.12] | 0.00001 | 93 | |
| Postoperative PCA use | |||||
| Yes | 3 | 314.49 [276.22, 352.77] | 0.00001 | 0 | 0.02 |
| No | 9 | 236.52 [186.24, 286.80] | 0.00001 | 93 | |
| DEX dosage | |||||
| ≤1 µg/kg | 8 | 213.20 [169.44, 256.96] | 0.00001 | 89 | 0.0001 |
| >1 µg/kg | 4 | 329.21 [297.12, 361.30] | 0.00001 | 3 | |
| Anesthesia | |||||
| General | 10 | 271.55 [217.40, 325.71] | 0.00001 | 93 | 0.40 |
| Spinal | 2 | 199.07 [40.40, 357.74] | 0.01 | 93 | |
DEX, dexmedetomidine; PCA, patient-controlled analgesia; CI: confidence interval.
The GRADE evidence profiles for the outcome measures are shown in Table 4. The level of evidence was moderate for pain intensity at rest at postoperative 1 h, and low for pain intensity on movement at postoperative 1 h, morphine-equivalents at postoperative 0–24 h, time to first request for postoperative analgesia, PONV, hypotension, and bradycardia.
Table 4.
GRADE evidence profile.
| Quality assessment | № of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | DEX | Control | Relative (95% CI) | Absolute (95% CI) | ||
| Pain intensity at rest at 1 h postoperatively | ||||||||||||
| 10 | randomised trials | not serious | seriousa | not serious | not serious | none | 256 | 238 | — | MD 1.57 lower (1.94 lower to 1.2 lower) |
⨁⨁⨁◯ MODERATE |
IMPORTANT |
| Pain intensity on movement at 1 h postoperatively | ||||||||||||
| 3 | randomised trials | seriousb | seriousc | not serious | not serious | none | 90 | 70 | — | MD 1.71 lower (2.39 lower to 1.03 lower) |
⨁⨁◯◯ LOW |
IMPORTANT |
| Morphine-equivalents 0–24 h (mg) | ||||||||||||
| 6 | randomised trials | not serious | very seriousd | not serious | not serious | none | 151 | 153 | — | MD 8.54 lower (11.96 lower to 5.13 lower) |
⨁⨁◯◯ LOW |
IMPORTANT |
| Time to first analgesic request (min) | ||||||||||||
| 12 | randomised trials | not serious | very seriouse | not serious | not serious | none | 316 | 278 | — | MD 257.57 higher (209.86 higher to 305.28 higher) |
⨁⨁◯◯ LOW |
IMPORTANT |
| Postoperative nausea and vomiting | ||||||||||||
| 12 | randomised trials | not serious | not serious | not serious | very serious f | none | 10/316 (3.2%) | 6/278 (2.2%) | RR 1.37 (0.52 to 3.62) |
8 more per 1,000
(from 10 fewer to 57 more) |
⨁⨁◯◯ LOW |
IMPORTANT |
| Hypotension | ||||||||||||
| 12 | randomised trials | not serious | not serious | not serious | very serious g | none | 8/316 (2.5%) | 2/278 (0.7%) | RR 2.20 (0.67 to 7.23) |
9 more per 1,000
(from 2 fewer to 45 more) |
⨁⨁◯◯ LOW |
IMPORTANT |
| Bradycardia | ||||||||||||
| 12 | randomised trials | not serious | not serious | not serious | very serious h | none | 10/316 (3.2%) | 3/278 (1.1%) | RR 2.20 (0.81 to 5.97) |
13 more per 1,000
(from 2 fewer to 54 more) |
⨁⨁◯◯ LOW |
IMPORTANT |
DEX, dexmedetomidine; CI, confidence interval; MD, mean difference; RR, risk ratio.
aHeterogeneity: I2 = 89%.
bAll the trials were judged to be at unclear risk of bias.
cHeterogeneity: I2 = 86%.
dHeterogeneity: I2 = 95%.
eHeterogeneity: I2 = 93%.
fRR with 95% CI: 3.00 (0.13–69.52) for one trial and 7.00 (0.38–129.93) for another.
gRR with 95% CI: 3.00 (0.13–69.52), 3.00 (0.13–70.83), and 7.00 (0.38–129.93) for three trials.
hRR with 95% CI: 1.54 (0.07–36.11), 3.00 (0.13–69.52), and 3.00 (0.13–70.83) for three trials.
Discussion
In this meta-analysis, intra-articular DEX use significantly decreased postoperative pain and opioid consumption in patients undergoing arthroscopic knee surgery. The analgesic effect of DEX was also indicated by a longer time to first request for postoperative analgesia. The evidence for each outcome measure was verified by TSA. No difference was found in the incidence of PONV, hypotension, or bradycardia between the DEX and control groups.
Arthroscopic surgery is a minimally invasive orthopedic surgical procedure. However, postoperative pain can be underestimated. Al-Metwalli et al. showed that patients treated with intra-articular and i.v. saline reported pain intensity of 5 points on a VAS up to 12 h postoperatively7. This pain, if left untreated, delays patient discharge and postoperative rehabilitation. Considering the adverse effects associated with systemic opioid use, intra-articular analgesia administration is simple and may provide a better alternative. In this meta-analysis, DEX treatment decreased VAS pain scores by 1.57 points at rest and 1.71 points on movement at postoperative 1 h. The reduction in postoperative pain decreased to 0.34 points at 24 h, but the difference remained significant. Further, morphine-equivalents were 8.54 mg lower and the time to first request for postoperative analgesia was 257.57 min longer in the DEX treatment group compared to the control group. To the authors’ knowledge, these are the first data to demonstrate that the analgesic effects of intra-articular DEX occur mainly in the early postoperative period.
The mechanism underlying the effects of intra-articular DEX is unknown. There may be direct local action, but central action by systemic absorption should not be excluded. A recent study revealed simultaneous perineural administration of clonidine and DEX prolonged sensory and motor blockade by local anesthetics32,33. Supraspinal, spinal, and peripheral mechanisms could be involved in the analgesic effects of α2 adrenergic receptor agonists34. Similar to clonidine, DEX acts on presynaptic receptors and inhibits the release of norepinephrine at peripheral afferent nociceptors6. Some evidence suggests that α2 adrenoceptor stimulation causes analgesia by facilitating inhibitory synaptic responses in the superficial dorsal horn35. Notably, the effects of DEX are not limited to its interactions with α2 adrenergic receptors. A direct inhibition of tetrodotoxin-resistant Na+ channels may contribute to the antinociceptive effects of clonidine and DEX when used in addition to local anesthesia36. Another study indicated that DEX inhibited neuronal delayed-rectifier potassium currents and sodium currents to produce local anesthetic effects37.
Systemic DEX infusion potentiates intra- and postoperative analgesia, and perineural DEX may improve the onset and quality of neuraxial and peripheral nerve block33,38. However, hemodynamic changes such as hypotension, bradycardia, or both have been reported. In this meta-analysis, intra-articular DEX did not increase the incidence of hypotension or bradycardia compared to that in the control group. The low incidence of adverse effects may be related to the lack of vessels in the articular surface and the relatively small dose of DEX administered.
This meta-analysis has several limitations. First, only 12 trials met the inclusion criteria, and the number of patients was relatively small. Second, substantial heterogeneity was found for some outcome measures. According to subgroup analyses, inconsistencies in the use of local anesthetic and in the postoperative PCA and DEX doses may account for the heterogeneity. Third, although TSA indicated sufficient evidence for the conclusions, the level of evidence achieved by the GRADE methodology was low or moderate. Therefore, the current results should be interpreted with caution. Finally, there are no data on the long-term effects of intra-articular DEX administration; therefore, this study is only relevant to the early postoperative period, specifically, up to postoperative 24 h. Consequently, further well-conducted prospective studies with adequate power to evaluate short-and long-term outcomes after intra-articular administration of DEX are warranted.
In conclusion, intra-articular administration of DEX improved pain outcomes in the early postoperative period after knee arthroscopy. More evidence is required to confirm these findings.
Electronic supplementary material
Acknowledgements
This work was supported, in part, by grants from the National Natural Science Foundation of China (81471835 and 81671880 to F.-H.J., 81601666 to J.Z., and 81601659 to K.P.) and the Jiangsu Provincial Medical Youth Talents Program (QNRC2016741 to K.P.)
Author Contributions
Peng, K: Design of the study, acquisition of data, analysis and interpretation of data, drafting and revising the manuscript, approval of the final version. Chen, W.R.: Acquisition of data, analysis and interpretation of data, approval of the final version. Meng, X.W.: Acquisition of data, analysis and interpretation of data, approval of the final version. Zhang, J.: Acquisition of data, approval of the final version. Ji, F.H.: Design of the study, revising the manuscript, approval of the final version.
Competing Interests
The authors declare no competing interests.
Footnotes
Ke Peng and Wei-rong Chen contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-22482-8.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. The Journal of bone and joint surgery. American volume. 2011;93:994–1000. doi: 10.2106/JBJS.I.01618. [DOI] [PubMed] [Google Scholar]
- 2.Gerlach AT, Murphy CV, Dasta JF. An updated focused review of dexmedetomidine in adults. The Annals of pharmacotherapy. 2009;43:2064–2074. doi: 10.1345/aph.1M310. [DOI] [PubMed] [Google Scholar]
- 3.Dahl MR, Dasta JF, Zuelzer W, McSweeney TD. Lidocaine local anesthesia for arthroscopic knee surgery. Anesth Analg. 1990;71:670–674. doi: 10.1213/00000539-199012000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Stein C, et al. Analgesic effect of intraarticular morphine after arthroscopic knee surgery. N Engl J Med. 1991;325:1123–1126. doi: 10.1056/NEJM199110173251602. [DOI] [PubMed] [Google Scholar]
- 5.Bondok RS, Abd El-Hady AM. Intra-articular magnesium is effective for postoperative analgesia in arthroscopic knee surgery. Br J Anaesth. 2006;97:389–392. doi: 10.1093/bja/ael176. [DOI] [PubMed] [Google Scholar]
- 6.Gentili M, Juhel A, Bonnet F. Peripheral analgesic effect of intra-articular clonidine. Pain. 1996;64:593–596. doi: 10.1016/0304-3959(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 7.Al-Metwalli RR, et al. Effect of intra-articular dexmedetomidine on postoperative analgesia after arthroscopic knee surgery. Br J Anaesth. 2008;101:395–399. doi: 10.1093/bja/aen184. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins, J. & Green, S. Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration Version 5.1.0, Available from: www.cochrane-handbook.org (2011).
- 10.Turan A, et al. Preoperative angiotensin-converting enzyme inhibitor use is not associated with increased postoperative pain and opioid use. Clin J Pain. 2013;29:1050–1056. doi: 10.1097/AJP.0b013e318287a258. [DOI] [PubMed] [Google Scholar]
- 11.Von Korff M, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24:521–527. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guyatt GH, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 14.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ning GZ, et al. Rivaroxaban for thromboprophylaxis after total hip or knee arthroplasty: a meta-analysis with trial sequential analysis of randomized controlled trials. Sci Rep. 2016;6:23726. doi: 10.1038/srep23726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu X, et al. Tomato consumption and prostate cancer risk: a systematic review and meta-analysis. Sci Rep. 2016;6:37091. doi: 10.1038/srep37091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive–Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol. 2009;38:287–298. doi: 10.1093/ije/dyn188. [DOI] [PubMed] [Google Scholar]
- 19.Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008;61:64–75. doi: 10.1016/j.jclinepi.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med. 2001;135:982–989. doi: 10.7326/0003-4819-135-11-200112040-00010. [DOI] [PubMed] [Google Scholar]
- 21.Alipour M, et al. Effect of dexmedetomidine on postoperative pain in knee arthroscopic surgery; a randomized controlled clinical trial. The archives of bone and joint surgery. 2014;2:52–56. [PMC free article] [PubMed] [Google Scholar]
- 22.Cao, W. B. & Liu, Z. F. Clinical observation of intraarticular dexmedtomidine on postoperative analgesia after arthroscopic knee surgery. Jilin Medicine. 37, 312–314 [article in Chinese] (2016).
- 23.Cui, J. Z., Zhang, X. B., Zhang, Y. H. & Zhao, Z. B. Efficacy of intra-articular dexmedetomidine for postoperative analgesia in arthroscopic knee surgery. Chinese Journal of Modern Medicine. 24, 94–97 [article in Chinese] (2014).
- 24.Elbadawy AM, Salama AK, Mohammad MM. Comparative study of intra-articular dexmedetomidine versus ketamine as adjuvant analgesics after knee arthroscopy. Egyptian J Anaesth. 2015;31:309–314. doi: 10.1016/j.egja.2015.05.003. [DOI] [Google Scholar]
- 25.Lei, L. et al. Efficacy of intra-articuIar dexmedetomidine for postoperative analgesia in patients with arthroscopic knee surgery. Pain Clin J. 10, 121–124 [article in Chinese] (2014).
- 26.Panigrahi R, et al. Intra-articular adjuvant analgesics following knee arthroscopy: comparison between single and double dose dexmedetomidine and ropivacaine a multicenter prospective double-blind trial. Orthop Surg. 2015;7:250–255. doi: 10.1111/os.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panigrahi R, et al. High dose dexamethasone offers better postoperative analgesia than dexmedetomidine when added to intra articular ropivacaine following knee arthroscopic surgery. Anaesth, Pain & Intensive Care. 2016;20:273–277. [Google Scholar]
- 28.Paul S, Bhattacharjee DP, Ghosh S, Dawn S, Chatterjee N. Efficacy of intra-articular dexmedetomidine for postoperative analgesia in arthroscopic knee surgery. Ceylon Med J. 2010;55:111–115. doi: 10.4038/cmj.v55i4.2627. [DOI] [PubMed] [Google Scholar]
- 29.Wang, C. G. et al. The efficacy of dexmedetomidine for multimodal analgesia on postoperative analgesia after arthroscopic knee surgery. Int J Anesth Resus. 35, 977–980 [article in Chinese] (2014).
- 30.Yao, M. et al. The effects of intra-articular infusion dexmedetomidine on postoperative analgesia in patients with arthroscopic knee surgery. J Clin Anesthesiol. 28, 896–898 [article in Chinese] (2012).
- 31.Zhang, Y., Yin, J. L., Wang, X. L., Bao, H. G. & Chen, L. H. Efficacy of Intra-articular dexmedetomidine in combination with ropivacaine for postoperative analgesia in arthroscopic knee surgery. Pharmaceutical and Clinical Research. 23, 294–296 [article in Chinese] (2015).
- 32.Kirksey MA, Haskins SC, Cheng J, Liu SS. Local Anesthetic Peripheral Nerve Block Adjuvants for Prolongation of Analgesia: A Systematic Qualitative Review. PLoS One. 2015;10:e0137312. doi: 10.1371/journal.pone.0137312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdallah FW, Brull R. Facilitatory effects of perineural dexmedetomidine on neuraxial and peripheral nerve block: a systematic review and meta-analysis. Br J Anaesth. 2013;110:915–925. doi: 10.1093/bja/aet066. [DOI] [PubMed] [Google Scholar]
- 34.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–394. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Funai Y, et al. Systemic dexmedetomidine augments inhibitory synaptic transmission in the superficial dorsal horn through activation of descending noradrenergic control: an in vivo patch-clamp analysis of analgesic mechanisms. Pain. 2014;155:617–628. doi: 10.1016/j.pain.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oda A, et al. Effects of alpha2-adrenoceptor agonists on tetrodotoxin-resistant Na+ channels in rat dorsal root ganglion neurons. Eur J Anaesthesiol. 2007;24:934–941. doi: 10.1017/S0265021507000543. [DOI] [PubMed] [Google Scholar]
- 37.Chen BS, Peng H, Wu SN. Dexmedetomidine, an alpha2-adrenergic agonist, inhibits neuronal delayed-rectifier potassium current and sodium current. Br J Anaesth. 2009;103:244–254. doi: 10.1093/bja/aep107. [DOI] [PubMed] [Google Scholar]
- 38.Vorobeichik L, Brull R, Abdallah FW. Evidence basis for using perineural dexmedetomidine to enhance the quality of brachial plexus nerve blocks: a systematic review and meta-analysis of randomized controlled trials. Br J Anaesth. 2017;118:167–181. doi: 10.1093/bja/aew411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.