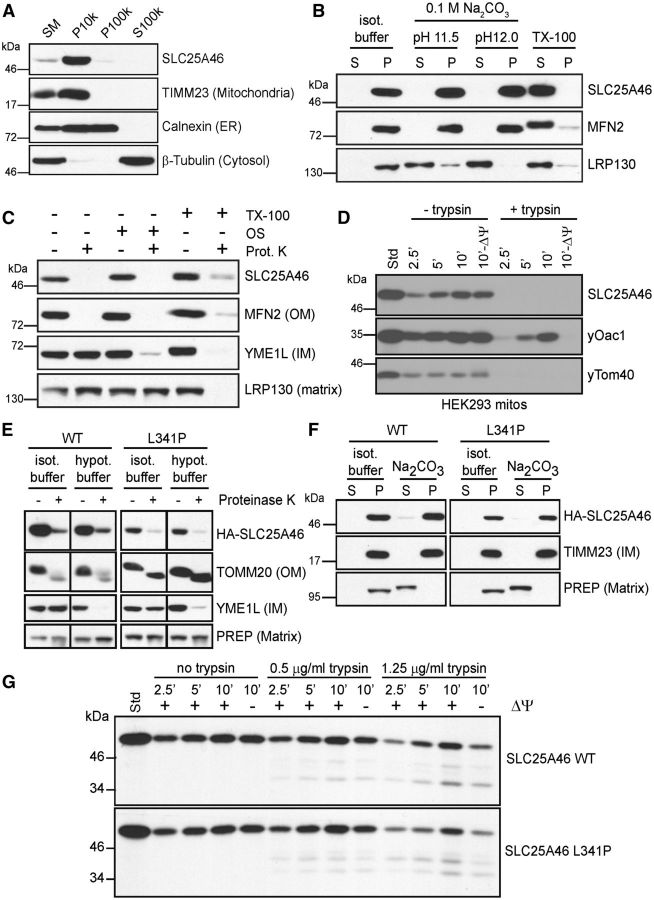
Figure 3.
Localization of SLC25A46 to the outer mitochondrial membrane. (A) Subcellular fractionation of LAN5 cells by differential centrifugation. SM = starting material, P10k = 10 000g pellet, P100k = 100 000g pellet, S100k = 100 000g post-supernatant. Selected antibodies detected SLC25A46, TIMM23 (mitochondria), calnexin (ER, endoplasmic reticulum), and β-tubulin (cytosol). (B) Alkaline extraction in 0.1 M Na2CO3 solution at pH11 and 12 was performed on mitochondria isolated from LAN5 cells. The samples were separated into pellet (P) and supernatant (S) fractions. 1% Triton™ X-100 (TX-100) treatment and the starting material incubated in isotonic buffer were included as controls. Selected antibodies detected SLC25A46, MFN2 (outer membrane), and LRP130 (matrix). (C) Sub-mitochondrial localization was tested by osmotic shock (OS). After lysis of mitochondria in hypotonic buffer, the pellet fraction was collected and subject to proteinase K treatment. As a control, intact mitochondria were treated with protease and the pellet from the osmotic shock fraction was treated with 1% Triton™ X-100. Selected antibodies detected SLC25A46, MFN2 (OM, outer membrane), YME1L (IM, inner membrane), and LRP130 (matrix). (D) Import of radiolabelled SLC25A46, yeast Oac1 (IM), and yeast Tom40 (OM) into HEK293T mitochondria followed by subsequent trypsin treatment as indicated and alkaline extraction. The membrane potential was disrupted by CCCP (−ΔΨ). (E) As in C, isolated mitochondria from HEK293T cells stably expressing 2 × HA-SLC245A46 wild-type (WT) or L341P were treated with proteinase K in isotonic or hypotonic buffer to determine the sub-mitochondrial localization of wild-type and mutant SLC25A46. Controls include TOMM20 (OM), YME1L (IM) and PreP (matrix). (F) As in B, alkaline extraction (pH 12) on isolated mitochondria from stable HEK239T cells expressing 2 × HA-SLC245A46 wild-type (WT) or L341P. (G) Import of radiolabelled wild-type SLC25A46 or L341P mutant into HEK293T mitochondria followed by carbonate extraction and trypsin treatment. CCCP was used to disrupt the membrane potential (−ΔΨ).