Many symptoms in behavioural variant frontotemporal dementia (bvFTD) reflect impairment in reward processing. In a series of laboratory paradigms, Perry et al. demonstrate that patients with bvFTD show selective insensitivity to olfactory stimuli that controls find aversive. These changes in valence judgements correlate with amygdala and insula atrophy.
Keywords: frontotemporal dementia, reward, psychophysiology
Abstract
During reward processing individuals weigh positive and negative features of a stimulus to determine whether they will pursue or avoid it. Though patients with behavioural variant frontotemporal dementia display changes in their pursuit of rewards, such as food, alcohol, money, and sex, the basis for these shifts is not clearly established. In particular, it is unknown whether patients’ behaviour results from excessive focus on rewards, insensitivity to punishment, or to dysfunction in a particular stage of reward processing, such as anticipation, consumption, or action selection. Our goal was to determine the nature of the reward deficit in behavioural variant frontotemporal dementia and its underlying anatomy. We devised a series of tasks involving pleasant, unpleasant, and neutral olfactory stimuli, designed to separate distinct phases of reward processing. In a group of 25 patients with behavioural variant frontotemporal dementia and 21 control subjects, diagnosis by valence interactions revealed that patients with behavioural variant frontotemporal dementia rated unpleasant odours as less aversive than did controls and displayed lower skin conductance responses when anticipating an upcoming aversive odour. Subjective pleasantness ratings and skin conductance responses did not differ between behavioural variant frontotemporal dementia and controls for pleasant or neutral smells. In a task designed to measure the effort subjects would expend to smell or avoid smelling a stimulus, patients with behavioural variant frontotemporal dementia were less motivated, and therefore less successful than control subjects, at avoiding what they preferred not to smell, but had equivalent success at obtaining stimuli they found rewarding. Voxel-based morphometry of patients with behavioural variant frontotemporal dementia revealed that the inability to subjectively differentiate the valence of pleasant and unpleasant odours correlated with atrophy in right ventral mid-insula and right amygdala. High pleasantness ratings of unpleasant stimuli correlated with left dorsal anterior insula and frontal pole atrophy. These findings indicate that insensitivity to negative information may be a key component of the reward-seeking behaviours in behavioural variant frontotemporal dementia, and may relate to degeneration of structures that are involved in representing the emotional salience of sensory information.
Introduction
Reward processing is altered in patients with behavioural variant frontotemporal dementia (bvFTD), as seen in changes in their pursuit of alcohol, sex, food, money, and social engagement (Miller et al., 1995; Perry et al., 2014; Perry and Kramer, 2015). While patients frequently display apathy, they may also show a selective increased pursuit of some rewards. This increased reward-seeking has been linked to atrophy in structures that are components of the reward circuit, including the ventral striatum and insula (Whitwell et al., 2007; Woolley et al., 2007; Perry et al., 2014). Patients with bvFTD also overspend (Chiong et al., 2014), make risky monetary gambles (Rahman et al., 1999; Torralva et al., 2007), and have been shown to be more motivated to seek monetary gain than to avoid monetary loss (Perry et al., 2015). It is unknown whether these behaviours relate to increased motivation for reward or decreased concern about negative consequences. It has also not been established in bvFTD at what stage in the process of evaluating rewards the breakdown occurs. Reward processing involves multiple stages, including anticipation of reward, motivated action to obtain the reward, receipt or consumption of reward, and an update in perceived value in the form of a reward prediction error or salience update. These different stages have been linked to different neuroanatomy, including reward consumption with ventromedial prefrontal cortex and reward anticipation with the ventral striatum (Diekhof et al., 2012).
Our objective was to identify the abnormal aspect of reward processing in bvFTD and identify its anatomical correlates. Because common monetary reward paradigms may either be too complicated for patients with neurodegenerative disease (and can be failed for reasons that relate to cognitive impairment rather than reward) (Ernst et al., 2002; Manes et al., 2002) and simpler, more primary rewards may be more salient for patients, we designed a series of reward experiments involving the sense of smell. Olfactory stimuli elicit activation in the same regions as rewards in other sensory modalities (Zald and Pardo, 1997; O'Doherty et al., 2000; Royet et al., 2000; Rolls et al., 2003) and induce measurable physiological responses (Alaoui-Ismaïli et al., 1997; Bensafi et al., 2002). We hypothesized that patients with bvFTD would respond less to negative olfactory stimuli than control subjects, and that this would relate to reward circuit atrophy, including the insula, which has been linked to processing of negative information (Seymour et al., 2007; Samanez-Larkin et al., 2008).
Materials and methods
Subjects
Patients with bvFTD and normal controls were recruited to participate in the task. All subjects underwent evaluation as part of research studies at the University of California San Francisco. Patients with bvFTD all met at least possible bvFTD diagnostic criteria (Rascovsky et al., 2011) based on the consensus determination of multidisciplinary UCSF Memory and Aging Center clinicians. Written informed consent was obtained from patients or surrogates according to procedures approved by the UCSF Committee on Human Research. As part of their evaluation, all subjects underwent neuropsychological assessment with a battery including tests of memory, language, visuospatial abilities, and executive function. Control subjects were screened for the presence of any neurological or psychiatric disorder. The severity of functional impairment in patients was also assessed using the Clinical Dementia Rating scale (CDR) (Morris, 1993), and those with scores >2 (indicative of more than moderate dementia severity) were excluded from the study as they were unlikely to be able to perform the task correctly. A total of 25 patients with bvFTD and 21 controls completed the task and were included in the analysis (Table 1).
Table 1.
Group demographics
| Control | bvFTD | Statistical comparison | |
|---|---|---|---|
| n = 21 | n = 25 | ||
| Gender (male/female) | 5/16 | 16/9 | χ2(1, n = 46) = 5.90, P = 0.015 |
| Age | 61.7 (10.1) | 63.9 (8.4) | t(44) = 0.81, P = 0.42 |
| MMSE | - | 24.4 (3.5) | |
| CDR-SB | - | 6.5 (2.9) |
Results displayed as mean (standard deviation) for age, MMSE, and CDR-SB. CDR-SB = Clinical Dementia Rating Scale Sum of Boxes; MMSE = Mini-Mental State Examination.
Reward task procedure
Participants were seated in front of a computer screen and underwent a series of tasks designed to evaluate three stages of reward processing: reward consumption, reward anticipation, and effort to obtain reward or avoid punishment. All tasks involved the sequential presentation of a series of seven olfactants in glass vials. Pleasant, or positive valence olfactants included vanillin (8% in propylene glycol), menthol (10% in propylene glycol), and citral (10% in propylene glycol). Unpleasant, or negative valence olfactants included isovaleric acid (5% in propylene glycol), propionic acid (1% in propylene glycol), and pyridine (1% in propylene glycol). Propylene glycol (100%) was used as a neutral stimulus. Olfactants were obtained from Sigma-Aldrich. The olfactants selected have been used in prior studies and have consistently been rated by healthy controls as having the desired positive or negative valence (Alaoui-Ismaïli et al., 1997; Bensafi et al., 2002; Anderson et al., 2003; Rolls et al., 2003).
Tasks were administered in E-prime version 2.0. When cued by the programme the participant would inhale, exhale, and then sniff for 3 s while the experimenter placed a glass vial beneath the participant’s nose. Skin conductance response (SCR) was obtained during the tasks, measured in microSiemens (µS) on a continuous basis via two 1081 FG-DIN Ag/AgCl sensors prepared with Biogel electrode gel (UFI Inc.) attached to the ventral surface of the middle phalanges on the middle and index fingers (eight controls and eight with bvFTD) or index and ring fingers (eight controls and 13 with bvFTD) of the non-dominant hand. There were no significant differences in within diagnosis age, gender, or distribution of diagnoses between the two electrode placement configurations. Physiological signals were recorded using a James Long amplifier and Biopac amplifiers recorded through a Biopac MP150 data acquisition unit using AcqKnowledge software (version 4.2, www.biopac.com). Given their typically skewed distribution, SCR values were log transformed for normalization (Society for Psychophysiological Research Ad Hoc Committee on Electrodermal Measures, 2012). Statistics were carried out in R (R Core Team, 2015).
Reward consumption task
Three pleasant, three unpleasant, and one neutral stimulus were presented in random order. Subjects were asked to smell each one for 3 s and then to rate the pleasantness of each on a 1–9 scale with 1 representing very unpleasant and 9 being extremely pleasant, with 5 being neutral. Stimuli were only presented once and at an interval of 74 s to allow olfactants to dissipate and prevent olfactory habituation associated with repetitive stimulus presentation. SCR was measured as the difference between the maximum value in the 10 s after smelling the stimulus and the mean value in the 1 s baseline preceding the pre-sniff cue to inhale.
Reward anticipation task
Subjects were presented with the same olfactory stimuli as in the previous task, but in this task each was preceded by a description on the screen of the upcoming smell. These descriptions used familiar, colloquial labels rather than scientific terminology in order to evoke a response based upon anticipated valence (vanillin = vanilla, citral = lemon, menthol = mint, isovaleric acid = sweaty feet, pyridine = fish, propionic acid = vinegar, propylene glycol = no smell). There were two rounds, with each of the seven smells presented in randomized order one time in each round, with an interval of 47 s between each smell. SCR was assessed during two periods, an anticipation period of 8 s following presentation of the upcoming odour description, and a response period of 10 s after sniffing the stimulus. For both periods, SCR was measured as the maximum value in the period minus the mean value at baseline (1 s immediately preceding cue presentation). Sixteen patients with bvFTD and 21 normal controls had valid and complete SCR recordings and were included in this analysis. As task performance depended on understanding written cues, to assess for any effect of semantic impairment on the results, we also performed a subgroup analysis excluding patients who showed any sign of word reading or single-word comprehension deficits.
Effort to obtain reward task
After sequential presentation of the same on-screen descriptive cues from the reward anticipation task, subjects chose by button press whether they wanted to smell each odorant or not. Subjects were instructed to press either a button marked ‘yes’ or one marked ‘no’ as quickly as they could, and they were told that success depended on how quickly they pushed the button. Based upon the rate of pressing either button during the 5 s following their decision they successfully received their preference or not. To account for individual or disease-related factors that could affect motor speed, each subject’s threshold number of button presses was derived from that subject’s performance on a practice trial. The threshold for each of the seven trials was 10% more than the number of button presses during practice. For example, if the cue was ‘vanilla’ and the subject exceeded the necessary threshold number of button presses he or she would then be presented with vanillin, and if the number of presses did not reach threshold no stimulus would be given. Subjects were not informed of their specific threshold or of how the threshold was established. A subset of patients (11 controls and six with bvFTD) completed this task. Age, gender, Mini-Mental State Examination (MMSE), and CDR sum of boxes (CDR-SB) did not differ significantly between subjects who completed this task and those who did not. Patients who completed this task showed no sign of impairment in word reading or single word comprehension.
Odour discrimination task
To assess the integrity of participants’ sense of smell we presented each patient with 10 pairs of smells (using the same seven olfactants) and asked them if these two smells were the same or different from each other. This control task allowed us to distinguish findings that relate to reward from those that could pertain to olfactory acuity.
Image acquisition
Of the patients included, 24 with bvFTD had useable neuroimaging performed within 6 months of the time of reward testing. MRI images were acquired at the UCSF Neuroscience Imaging Center on a 3 T Siemens TIM Trio scanner equipped with a 12-channel head coil. Whole brain images were acquired (MPRAGE; repetition time/echo time/inversion time = 2300/2.98/900 ms, 9° flip angle). The field of view was 240 × 256 mm, with 1 × 1 mm in-plane resolution and 1 mm slice thickness.
Imaging analysis
Voxel-based morphometry (VBM) was performed on bvFTD patient scans using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/). Preprocessing included segmentation into grey and white matter, alignment and normalization to Montreal Neurological Institute (MNI) space, modulation and smoothing with an 8 mm full-width at half-maximum Gaussian kernel. Multiple regression was performed to assess for areas of lower grey matter volume associated with valence ratings of pleasant and unpleasant olfactants. Because we were particularly interested in the relationship between perception of pleasant and unpleasant olfactants, and not just their individual ratings, we also performed multiple regression to assess for regions of low volume associated with a difference score derived from subtracting mean valence ratings of pleasant and unpleasant stimuli. Sex and total intracranial volume were covariates in each regression. To explore any effect of patient age or bvFTD disease severity on the imaging findings we correlated age and CDR-SB with the valence difference score. To focus on the hypothesized reward-relevant anatomical regions and in order to maximize power by reducing the effect of multiple comparisons, we masked the analysis to structures in the reward circuit: orbitofrontal cortex, insula, anterior cingulate cortex, caudate, putamen, globus pallidus, amygdala, and thalamus. The threshold for statistical significance was set at P < 0.05 after family-wise error (FWE) correction for multiple comparisons. Statistical maps were examined at a level of P < 0.001 uncorrected for multiple comparisons.
Results
Reward task performance
Reward consumption task
Self-reported valence
The main analysis was a mixed model ANOVA (controlling for age and sex) with diagnosis as a between-subjects factor, odour valence (pleasant, unpleasant, or neutral) as the within-subjects factor, and subjective pleasantness ratings of the seven stimuli as the dependent variable. As expected, given results of prior studies with these stimuli, there was a significant main effect of odour valence, with different pleasantness ratings among pleasant, unpleasant, and neutral odorants [F(2,88) = 42.2, P < 0.001]. There was no main effect of diagnosis (P = 0.72). There was a significant odour valence × diagnosis interaction [F(2,88) = 9.44, P < 0.001]. Consistent with our hypothesis, post hoc pairwise t-tests with Bonferroni correction revealed that patients with bvFTD rated unpleasant smells less negatively [t(136) = 3.19, P < 0.05] than controls but did not differ significantly in their rating of pleasant (P = 0.39) or neutral (P = 1) smells (Fig. 1).
Figure 1.
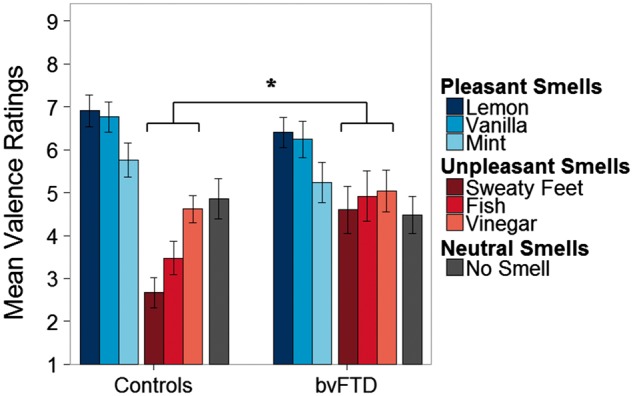
Pleasantness ratings for each smell by diagnosis showing a diagnosis × valence (pleasant, unpleasant, or neutral) interaction. Patients with bvFTD rated unpleasant smells as less aversive compared to controls (*P < 0.05). Pleasantness was rated 1–9 with 1 being extremely unpleasant and 9 extremely pleasant.
Skin conductance response
In a mixed model ANOVA (controlling for age and sex) with SCR as the dependent variable, the main effects of odour valence (P = 0.60) and diagnosis (P = 0.39) as well as the odour valence × diagnosis interaction (P = 0.18) were not significant, indicating that physiological responses did not differ between the groups during the consumption stage.
Reward anticipation task
Anticipation period
The main analysis was a mixed model ANOVA (controlling for age and sex) with diagnosis as a between-subjects factor, odour valence and round (first or second time through each stimulus) as within-subjects factors, and SCR as the dependent variable. There was a significant main effect of round [F(1,36) = 12.68, P < 0.05, lower SCR after receiving anticipation cues during the second round than when stimuli were more novel in the first round], but non-significant main effects of diagnosis (P = 0.45) and odour valence (P = 0.45). There was a significant diagnosis × odour valence interaction [F(2,144) = 3.41, P < 0.05] with post hoc tests indicating that patients with bvFTD had a significantly smaller SCR than controls when anticipating an unpleasant smell [t(220) = 2.94, P < 0.05], but no difference when anticipating pleasant (P = 1) or neutral (P = 1) smells (Fig. 2).
Figure 2.
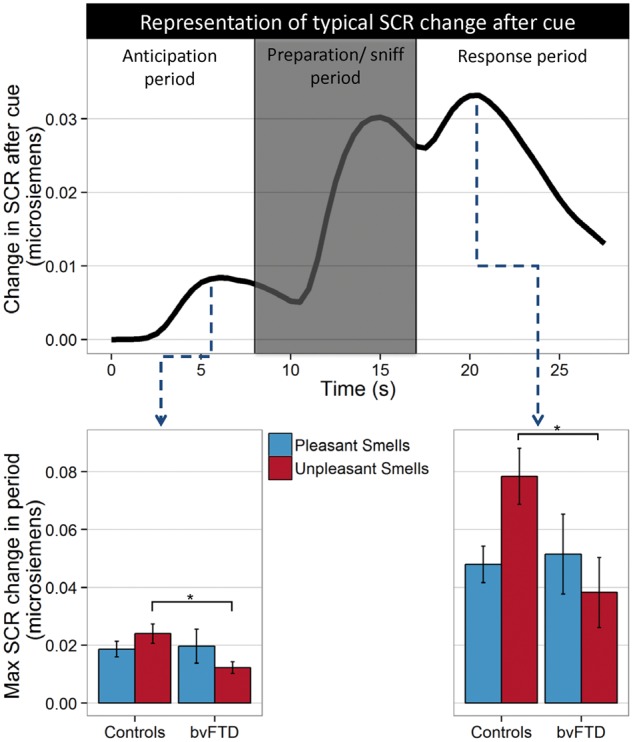
Skin conductance response (SCR) during reward anticipation task. Top: Typical SCR across one trial shows a peak after presentation of the anticipatory cue, followed by an increase related to the breathing in preparation for and during presentation of the smell, and then a final peak during the response period after smelling the stimulus. Bottom: Maximum SCR during each period by diagnosis. *P < 0.05.
Response period
SCR in the anticipation period strongly correlated with SCR in the response period (r = 0.8, P < 0.001), suggesting a strong effect of anticipation on the subsequent response to each odorant. There were significant main effects of round [F(1,36) = 6.13, P < 0.05] and odour valence [F(2,144) = 4.93, P < 0.01], but not diagnosis (P = 0.66). As during the anticipation period there was a significant diagnosis × odour valence interaction [F(2,144) = 3.80, P < 0.05], with post hoc tests indicating that patients with bvFTD had a significantly smaller SCR than controls after smelling an unpleasant smell [t(220) = 2.45, P < 0.05], but not after smelling a pleasant (P = 1) or neutral (P = 1) smell (Fig. 2).
Reading comprehension analysis
Four patients were identified whose cognitive testing showed any evidence of either word reading impairment (Wide Range Achievement Test 4 reading score <55/70 or missing any of the irregular words on the test) or poor single word comprehension (score of <13/16 on the 16-item modified version of the Peabody Picture Vocabulary Test – Revised). Exclusion of these four patients strengthened the significance of the diagnosis × valence interaction of SCR during both periods [F(2,132) = 3.92, P = 0.02 during anticipation and F(2,132) = 6.78, P = 0.002 in the response period], indicating that impaired comprehension of the written cues was unlikely to bias anticipation or response findings.
Effort to obtain reward task
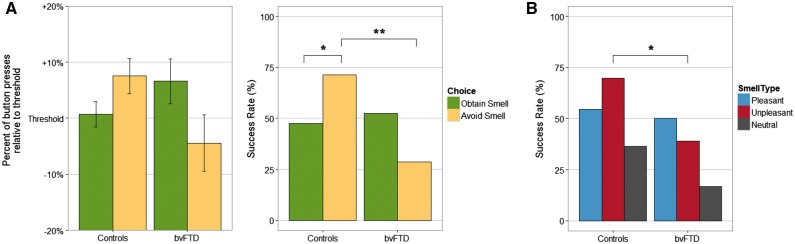
Patients with bvFTD and normal control subjects showed a trend towards a difference in how frequently they said ‘yes’ when asked if they wanted to smell each odour valence [72.2% of pleasant smells for bvFTD versus 90.9% for controls (χ2 = 3.08, P = 0.08), 38.9% versus 21.2% for unpleasant smells (χ2 = 1.83, P = 0.17), and 16.7% versus 45.5% for the neutral smell (χ2 = 1.41, P = 0.24)]. In a mixed model ANOVA comparing the per cent of threshold in button presses achieved for each trial, depending on their choice (to try to obtain or to avoid the smell) we found no main effect of diagnosis or choice. There was a significant diagnosis × choice interaction (P < 0.05, Fig. 3). Post hoc comparisons of each choice were non-significant, but they reflected a pattern of increased motivation in bvFTD to press the button to obtain what they wanted, as opposed to normal controls who were more motivated to work to avoid what they found aversive. This resulted in a significantly lower success rate at surpassing the threshold for bvFTD compared to controls when they chose to avoid the smell (χ2 = 9.75, P < 0.01, Fig. 3), but no difference when they chose to obtain it. When comparing success rates between bvFTD and normal controls at getting what they want for each smell type, patients with bvFTD were significantly less successful at obtaining their choice for unpleasant smells (χ2 = 4.56, P < 0.05, Fig. 3), with no difference for pleasant and neutral odours.
Figure 3.
Motivated action to obtain reward or avoid punishment. Subjects pressed a button depending on whether they chose to work to obtain or avoid a smell. The threshold for each subject was 10% more than the number of button presses during a practice trial. *P < 0.05; **P < 0.01. (A) Performance and success rate relative to threshold to obtain or avoid smells. (B) Success rate by smell type.
Odour discrimination
Nineteen patients with bvFTD and all 21 normal control subjects completed the odour discrimination task. Each participant received a discrimination score between 0 and 1 reflecting the relative frequency of correctly identifying whether odorant pairs were the same or different. Mean scores for normal controls (0.87) and patients with bvFTD (0.69) differed significantly [t(32.8) = 3.38, P = 0.002]. Among patients with bvFTD, discrimination scores did not correlate with mean pleasantness ratings (r = −0.03, P = 0.90), mean unpleasantness ratings (r = −0.16, P = 0.52), or the difference score between pleasant and unpleasant ratings (r = 0.22, P = 0.36), but did correlate with MMSE (Folstein et al., 1975) scores (r = 0.47, P = 0.04).
To explore whether inability to discriminate odours could influence how patients responded to olfactory stimuli, we performed three additional analyses of the self-reported pleasantness ratings from the reward consumption task:
Comparison of patients with low discrimination scores with those with high discrimination scores. About half of the patients with bvFTD (10/19) had a discrimination score ≥0.7. We compared pleasantness ratings for this high discrimination score group with those with scores ≤0.6 and found no significant effect of group (high- or low-scoring) and no group × odour valence interaction. Patients with intact odour discrimination rated pleasantness in the same way as those without.
Comparison of normal control subjects with only patients with high discrimination scores. The reported diagnosis × odour valence interaction from the reward consumption task remained significant when only including patients with discrimination scores ≥0.7, with some loss of power due to smaller sample size [F(2,54) = 4.32, P = 0.018].
Inclusion of discrimination score as a covariate. We ran the reward consumption task analysis of covariance for pleasantness ratings again with discrimination score included as a covariate. The diagnosis × odour valence interaction remained significant [F(2,76) = 7.76, P < 0.001].
As discrimination scores did not alter our findings, we included all patients in analyses, regardless of score.
Imaging
Difference between ratings of pleasant and unpleasant odours
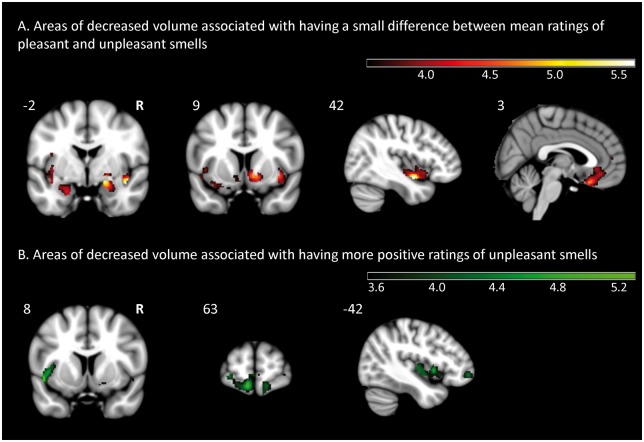
Brain regions in which volume loss correlated significantly with a smaller difference between subjective ratings of pleasant and unpleasant stimuli included the right ventral mid-insula (T = 5.64, PFWE < 0.05) and right amygdala (T = 5.57, PFWE < 0.05). Additional regions that exceeded a P < 0.001 threshold are reported in Table 2 and displayed in Fig. 4, and included the right ventral striatum, ventromedial prefrontal cortex, left insula, and left amygdala.
Table 2.
Anatomical correlates of valence ratings
| Anatomical region | Cluster volume (mm3) | Peak MNI coordinates | Maximum T score | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Difference score between mean rating of positive and negative valence stimuli (lower volume with smaller difference) | |||||
| Right insula* | 3159 | 42 | −2 | −14 | 5.64 |
| Right amygdala* | 948 | 20 | −2 | −12 | 5.57 |
| Left amygdala | 918 | −30 | 3 | −20 | 5.14 |
| Right pallidum | 1441 | 20 | −5 | −6 | 4.94 |
| Right nucleus accumbens | |||||
| Right putamen | |||||
| Right ventromedial prefrontal cortex | 5005 | 5 | 23 | −23 | 4.82 |
| Left ventromedial prefrontal cortex | |||||
| Bilateral anterior cingulate cortex | |||||
| Left insula | 1266 | −35 | 6 | −15 | 4.8 |
| Left rostromedial prefrontal cortex | 543 | −8 | 65 | −11 | 4.69 |
| Left superior orbitofrontal gyrus | 354 | −27 | 65 | −5 | 4.3 |
| Left middle orbitofrontal gyrus | |||||
| Left insula | 753 | −38 | 15 | −5 | 4.18 |
| Left middle orbitofrontal gyrus | 243 | −33 | 54 | −14 | 4 |
| Mean rating of unpleasant smells (lower volume with more positive ratings) | |||||
| Left insula | 3605 | −47 | 8 | −6 | 5.28 |
| Left rostromedial prefrontal cortex | 5694 | −8 | 63 | −14 | 5.02 |
| Left anterior cingulate cortex | 540 | 5 | 53 | 8 | 4.52 |
| Right anterior cingulate cortex | |||||
| Right medial superior frontal gyrus | |||||
| Right superior orbitofrontal gyrus | 756 | 12 | 65 | −14 | 4.31 |
| Right medial orbitofrontal gyrus | |||||
| Right rectal gyrus | |||||
| Right anterior cingulate cortex | 621 | 8 | 42 | 20 | 4.21 |
| Left anterior cingulate cortex | |||||
| Left superior medial frontal gyrus | |||||
| Right middle orbitofrontal gyrus | 250 | 36 | 57 | −2 | 4.14 |
| Right superior orbitofrontal gyrus | |||||
| Left anterior cingulate gyrus | 462 | −12 | 36 | 20 | 4 |
| Left superior medial frontal gyrus | |||||
*Significant at PFWE < 0.05. Cluster limit of 230 mm3.
Figure 4.
Voxel-based morphometry of regions associated with pleasantness ratings. Maps displayed at P < 0.001 uncorrected.
Mean ratings of unpleasant odours
While no regions survived FWE correction, the P < 0.001 uncorrected maps (Fig. 4 and Table 2) reveal that giving more positive ratings to unpleasant odours is associated with left dorsal insula and frontal pole atrophy.
Mean ratings of pleasant odours
No regions were significantly correlated with mean pleasantness ratings of positive valence odours either at a PFWE < 0.05 or P < 0.001 uncorrected level.
Age and disease severity analysis
To assess for any impact of age or bvFTD disease severity on the imaging findings, we correlated each of these in bvFTD patients with the mean ratings of unpleasant odours and with the difference score between pleasant and unpleasant ratings. There was no significant correlation for either age (r = 0.19, P = 0.36 for mean unpleasant ratings, r = −0.16, P = 0.46 for the valence difference score) or CDR-SB (r = 0.3, P = 0.15 for mean unpleasant ratings, r = −0.09, P = 0.68 for the valence difference score). Because of this lack of correlation, we did not include either as a covariate in the main analysis, but to verify the lack of impact on our imaging findings further, we also ran the main analysis of interest using age or CDR-SB as covariates. In the regression of the difference score between pleasant and unpleasant ratings, the associated regions and their effect sizes were minimally changed by the addition of either covariate, with some loss of statistical significance related to using an additional covariate with a small sample size. The strongest associated regions remained the right insula (T = 5.39, PFWE = 0.097 with age as covariate, T = 5.40, PFWE = 0.077 with CDR-SB as covariate) and right amygdala (T = 5.21, PFWE = 0.104 with age as covariate, T = 5.40, PFWE = 0.077 with CDR-SB as covariate).
Discussion
While the appropriate pursuit of reward is critical to health, personal success, and social life, aberrant approach and avoidance behaviour, as is seen in bvFTD, can have devastating consequences. In a series of laboratory tests, patients with bvFTD displayed insensitivity to unpleasant odorants in their behavioural and physiological responses. They subjectively rated unpleasant odorants as less aversive than control subjects did. They showed a diminished physiological response (SCR) when anticipating unpleasant smells, which then correlated with a diminished SCR after smelling the aversive stimuli. Their subjective and physiological responses to pleasant and neutral odours did not differ from those of control subjects. When given a choice to smell or avoid pleasant or unpleasant stimuli, and success at getting their choice was based on the rate of motivated button presses, patients with bvFTD were less successful for unpleasant smells than control subjects. In direct contrast to controls, they were particularly more motivated to obtain what they elected to smell than to avoid what they did not. Insensitivity to negative smells and loss of distinction between pleasant and unpleasant stimuli related to atrophy in reward-related structures, particularly the insula and amygdala. Loss of aversion to negative information may contribute to the profound behaviour changes in bvFTD.
Reward abnormalities in bvFTD have suggested that patients have an imbalanced perspective on reward and punishment. Patients have been more motivated by the prospect of monetary gain than loss (Rahman et al., 1999; Torralva et al., 2007; Perry et al., 2015). It has been unclear whether this imbalance is due to excessive drive for reward or insensitivity to punishment. This study indicates that lack of aversion to negative consequences is central to the reward abnormality in bvFTD.
Patients with bvFTD show reduced sensitivity to aversive things in other contexts. They often respond less to pain or extreme temperature (Fletcher et al., 2015a). Their response to disgusting stimuli is reduced (Eckart et al., 2012). They do not develop appropriate fear conditioning (Hoefer et al., 2008). They have trouble even recognizing negative emotion (Goodkind et al., 2015). They are less sensitive to negatively valenced contextual features in social decisions (Grossman et al., 2010). These behaviours could represent different facets of the same problem with reduced sensitivity to negative valence.
This series of tasks probed distinct stages of reward processing: reward consumption, anticipation, and motivated action to obtain reward. Patients with bvFTD demonstrated consistent deficits at processing negative valence across the stages. The lack of subjective aversion during the consumption stage, when experiencing a negative stimulus, may be fundamental to the deficits in other reward stages. During the reward consumption task, while subjective ratings differed, patients with bvFTD and controls did not differ in SCR from each other and there was no main effect of odour valence. This could either reflect a limitation in the olfactory stimuli at eliciting differing SCRs, or indicate that while patients with bvFTD may have an intact physiological response, they do not recognize or interpret this bodily signal in an appropriate manner. The fact that subjective pleasantness ratings differed from controls when SCR did not is consistent with a previously observed loss of coupling between autonomic responses and valence ratings for emotional sounds (Fletcher et al., 2015b). Decreased anticipation of unpleasant stimuli follows from the aberrant response to consumption. Anticipatory signals to a reward or punishment shift over time to reflect the actual consumption experience if it differs from prior expectation. This change in reward value may take the form of a reward prediction error (Schultz et al., 1997) or update of incentive salience (Berridge, 2012). In this case, patients with bvFTD mounted an appropriate anticipatory SCR based on how salient they expect the upcoming smell to be. SCR does not represent valence or expectation of valence, but corresponds to the level of arousal (Bensafi et al., 2002). In this case arousal was low in bvFTD when anticipating things that would normally be considered aversive, but patients’ recent experience would suggest otherwise. When assessing motivated action, patients with bvFTD exerted less effort to avoid than controls because their prior experience and anticipation indicated receiving the aversive smell would not be sufficiently punishing to motivate extra effort.
Inability to distinguish the valence differences in pleasant and unpleasant odours correlated with atrophy in the right ventral mid-insula and amygdala. The insula involvement could relate to its role in interoception, and without transmission of bodily cues of the environment or hedonic state from the insula to the striatum or anterior cingulate cortex an appropriate emotional response is not generated.
These findings provide additional insight into the evolving and contested conception of the amygdala’s role. Previously thought to only respond to aversive information, it was subsequently found to activate to positive and negative stimuli (Zald, 2003). Some prior tests of olfaction and gustation have suggested that the amygdala may be more relevant to processing intensity rather than stimulus valence (Anderson et al., 2003); however, it has also been found to discriminate both positive and negative from neutral odours independent of intensity (Winston et al., 2005), and to activate to increasing positive and negative valence even when arousal is controlled for (Anders et al., 2008). In this study the right amygdala seems to have been detecting stimulus reward salience. With degeneration patients had less ability to distinguish positive from negative and their ratings became more similar. This is particularly consistent with a prior study that showed that the right amygdala activates to extremes of valence in either the positive or negative direction (Phan et al., 2004). Interestingly, patients with bvFTD rated all three negative valence smells as slightly more positive than the neutral smell. They also elected to exert effort through button pressing to smell the aversive odours more often than they elected to work for the neutral stimulus. Stimuli of any salience were preferable to those without any.
At a lower significance threshold, atrophy of the right ventral striatum was also correlated with inability to distinguish valence differences. This is a key region in both anticipation and consumption of rewards (Diekhof et al., 2012).
Left dorsal anterior insula and frontal pole atrophy correlated with overly positive rating of unpleasant odours. While emotion lateralization models often attribute heightened negative emotion to a left hemisphere lesion, the link between insensitivity to negative information and atrophy of the regions observed in this study corresponds with some prior reports. A strikingly similar pattern of left dorsal insula and frontal pole atrophy correlated with the display of positive affect when viewing film clips (Sturm et al., 2015). Patients with more left dorsal anterior insula atrophy show less physiological reactivity to an aversive loud noise (Hoefer et al., 2008). The left dorsal anterior insula/opercular region responds preferentially to unpleasant compared to pleasant tastes (Small et al., 2003), suggesting that a lesion to this area would selectively impair response to the unpleasant. Integrity of regions including left insula/operculum correlates with an intact Behavioural Inhibition Scale (BIS) score, a marker of responsiveness to punishment, in bvFTD and Alzheimer’s disease (Shinagawa et al., 2015). These findings suggest a role of this left insula region in processing aversive information, and that degeneration of this area leads to insensitivity to the negative or excessive display of the positive.
Limitations of the study include reliance upon participants’ sense of smell. Olfaction deteriorates in neurodegenerative conditions such as Alzheimer’s and Parkinson’s disease (Mesholam et al., 1998), and has been called into question in bvFTD. Prior work has largely shown that patients with bvFTD have difficulty identifying odours (McLaughlin and Westervelt, 2008; Pardini et al., 2009; Omar et al., 2013; Heyanka et al., 2014; Magerova et al., 2014), possibly related to anomia or semantic problems, but have intact odour discrimination (Luzzi et al., 2007; Rami et al., 2007; Orasji et al., 2016). While patients with bvFTD in this study did not discriminate odours as well as the control subjects did, those with high discrimination scores rated valence no differently from those with low scores, and adding discrimination score as a covariate did not alter the significance of the diagnosis by odour valence interaction for subjective pleasantness ratings. It is unlikely that the integrity of the participants’ sense of smell influenced the findings, but results may vary across sensory modalities, as suggested by intact valence ratings of emotional sounds in bvFTD (Fletcher et al., 2015b).
Reward shifts in bvFTD encompass not only increased pursuit, but also profound apathy. We have previously observed (Perry et al., 2014) that increased reward-seeking behaviour may actually correlate with severity of apathy, indicating that the same patient may show an increase or decrease in motivation, depending on the stimulus. These seemingly conflicting motivational shifts could relate to differences in valuation of reward types, as processing of primary, abstract, or social rewards may involve different neuroanatomy (Rademacher et al., 2010; Sescousse et al., 2013). Task-specific factors could also suggest an alternative explanation for our observations. If the negative olfactory stimuli were more motivationally salient than the positive ones, the negative stimuli may have been more likely to show a shift of larger magnitude in the patients with bvFTD. While controls did not rate pleasant smells as closer to neutral than unpleasant smells, they did show more arousal by and effort to avoid unpleasant than pleasant smells. The performance of patients with bvFTD across the three trials was more consistent with a disproportionate effect on negative valence. Patients with bvFTD showed a selectively diminished response to unpleasant smells during anticipation and motivated effort. Their performance on pleasant smells was equivalent to controls. The evidence for a selective impairment in anticipating aversive smells was not just in comparison to controls, but in comparison to their own intact response to pleasant smells. Other study designs can further attempt to separate valence-specific changes in reward response from overall response tendencies or biases.
Though investigations of the disabling behaviour alterations in bvFTD have often focused on cognitive or social functioning deficits, reward processing provides an additional, less explored model for understanding these symptoms. In this study, we demonstrated a selective deficit for processing of negative olfactory stimuli in patients with bvFTD. Future studies can determine if analogous findings occur for other types of aversive or punishing experiences, or if paradigms using other reward types reveal differences in processing positive valence. Reward abnormalities occur in other neuropsychiatric diseases, including addiction, pathological gambling, eating disorders, mood disorders, schizophrenia, Parkinson’s disease, and autism. The link between lack of aversion to unpleasant stimuli or insensitivity to valence differences and lateralizing injury to the insula and amygdala could elucidate the mechanisms underlying behaviour changes in these conditions and the functions of these structures in health.
Funding
This study was supported by grants P01AG019724, K23AG045289, R01AG032306, R01AG022983, R01AG030688, R01AG052496, 1K23AG040127, and K24AG045333 from the NIH National Institute on Aging, and the Larry L. Hillblom Foundation.
Glossary
Abbreviations
- bvFTD
behavioural variant frontotemporal dementia
- SCR
skin conductance response
References
- Alaoui-Ismaïli O, Robin O, Rada H, Dittmar A, Vernet-Maury E. Basic emotions evoked by odorants: comparison between autonomic responses and self-evaluation. Physiol Behav 1997; 62: 713–20. [DOI] [PubMed] [Google Scholar]
- Anders S, Eippert F, Weiskopf N, Veit R. The human amygdala is sensitive to the valence of pictures and sounds irrespective of arousal: an fMRI study. Soc Cogn Affect Neurosci 2008; 3: 233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, et al. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci 2003; 6: 196–202. [DOI] [PubMed] [Google Scholar]
- Bensafi M, Rouby C, Farget V, Bertrand B, Vigouroux M, Holley A. Autonomic nervous system responses to odours: the role of pleasantness and arousal. Chem Senses 2002; 27: 703–9. [DOI] [PubMed] [Google Scholar]
- Berridge KC. From prediction error to incentive salience: mesolimbic computation of reward motivation. Eur J Neurosci 2012; 35: 1124–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiong W, Hsu M, Wudka D, Miller BL, Rosen HJ. Financial errors in dementia: testing a neuroeconomic conceptual framework. Neurocase 2014; 20: 389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Kaps L, Falkai P, Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude—an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia 2012; 50: 1252–66. [DOI] [PubMed] [Google Scholar]
- Eckart JA, Sturm VE, Miller BL, Levenson RW. Diminished disgust reactivity in behavioral variant frontotemporal dementia. Neuropsychologia 2012; 50: 786–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, et al. Decision-making in a risk-taking task: a PET study. Neuropsychopharmacology 2002; 26: 682–91. [DOI] [PubMed] [Google Scholar]
- Fletcher PD, Downey LE, Golden HL, Clark CN, Slattery CF, Paterson RW, et al. Pain and temperature processing in dementia: a clinical and neuroanatomical analysis. Brain 2015a; 138: 3360–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PD, Nicholas JM, Shakespeare TJ, Downey LE, Golden HL, Agustus JL, et al. Physiological phenotyping of dementias using emotional sounds. Alzheimers Dement 2015b; 1: 170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–98. [DOI] [PubMed] [Google Scholar]
- Goodkind MS, Sturm VE, Ascher EA, Shdo SM, Miller BL, Rankin KP, et al. Emotion recognition in frontotemporal dementia and Alzheimer's disease: a new film-based assessment. Emotion 2015; 15: 416–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Eslinger PJ, Troiani V, Anderson C, Avants B, Gee JC, et al. The role of ventral medial prefrontal cortex in social decisions: converging evidence from fMRI and frontotemporal lobar degeneration. Neuropsychologia 2010; 48: 3505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyanka DJ, Golden CJ, McCue RB 2nd, Scarisbrick DM, Linck JF, Zlatkin NI. Olfactory deficits in frontotemporal dementia as measured by the Alberta smell test. Appl Neuropsychol Adult 2014; 21: 176–82. [DOI] [PubMed] [Google Scholar]
- Hoefer M, Allison SC, Schauer GF, Neuhaus JM, Hall J, Dang JN, et al. Fear conditioning in frontotemporal lobar degeneration and Alzheimer's disease. Brain 2008; 131: 1646–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi S, Snowden JS, Neary D, Coccia M, Provinciali L, Lambon Ralph MA. Distinct patterns of olfactory impairment in Alzheimer's disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia 2007; 45: 1823–31. [DOI] [PubMed] [Google Scholar]
- Magerova H, Vyhnalek M, Laczo J, Andel R, Rektorova I, Kadlecova A, et al. Odor identification in frontotemporal lobar degeneration subtypes. Am J Alzheimers Dis Other Demen 2014; 29: 762–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, et al. Decision-making processes following damage to the prefrontal cortex. Brain 2002; 125: 624–39. [DOI] [PubMed] [Google Scholar]
- McLaughlin NC, Westervelt HJ. Odor identification deficits in frontotemporal dementia: a preliminary study. Arch Clin Neuropsychol 2008; 23: 119–23. [DOI] [PubMed] [Google Scholar]
- Mesholam RI, Moberg PJ, Mahr RN, Doty RL. Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer's and Parkinson's diseases. Arch Neurol 1998; 55: 84–90. [DOI] [PubMed] [Google Scholar]
- Miller BL, Darby AL, Swartz JR, Yener GG, Mena I. Dietary changes, compulsions and sexual behavior in frontotemporal degeneration. Dementia 1995; 6: 195–9. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43: 2412–4. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F, Kobal G, et al. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. Neuroreport 2000; 11: 893–7. [DOI] [PubMed] [Google Scholar]
- Omar R, Mahoney CJ, Buckley AH, Warren JD. Flavour identification in frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry 2013; 84: 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orasji SS, Mulder JL, de Bruijn SF, Wirtz PW. Olfactory dysfunction in behavioral variant frontotemporal dementia. Clin Neurol Neurosurg 2016; 141: 106–10. [DOI] [PubMed] [Google Scholar]
- Pardini M, Huey ED, Cavanagh AL, Grafman J. Olfactory function in corticobasal syndrome and frontotemporal dementia. Arch Neurol 2009; 66: 92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DC, Kramer JH. Reward processing in neurodegenerative disease. Neurocase 2015; 21: 120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DC, Sturm VE, Wood KA, Miller BL, Kramer JH. Divergent processing of monetary and social reward in behavioral variant frontotemporal dementia and Alzheimer disease. Alzheimer Dis Assoc Disord 2015; 29: 161–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DC, Sturm VE, Seeley WW, Miller BL, Kramer JH, Rosen HJ. Anatomical correlates of reward-seeking behaviours in behavioural variant frontotemporal dementia. Brain 2014; 137: 1621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho S, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage 2004; 21: 768–80. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. 2015.
- Rademacher L, Krach S, Kohls G, Irmak A, Gründer G, Spreckelmeyer KN. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage 2010; 49: 3276–85. [DOI] [PubMed] [Google Scholar]
- Rahman S, Sahakian BJ, Hodges JR, Rogers RD, Robbins TW. Specific cognitive deficits in mild frontal variant frontotemporal dementia. Brain 1999; 122 (Pt 8): 1469–93. [DOI] [PubMed] [Google Scholar]
- Rami L, Loy CT, Hailstone J, Warren JD. Odour identification in frontotemporal lobar degeneration. J Neurol 2007; 254: 431–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134: 2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Kringelbach ML, De Araujo IET. Different representations of pleasant and unpleasant odours in the human brain. Eur J Neurosci 2003; 18: 695–703. [DOI] [PubMed] [Google Scholar]
- Royet JP, Zald D, Versace R, Costes N, Lavenne F, Koenig O, et al. Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study. J Neurosci 2000; 20: 7752–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Hollon NG, Carstensen LL, Knutson B. Individual differences in insular sensitivity during loss anticipation predict avoidance learning. Psychol Sci 2008; 19: 320–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science 1997; 275: 1593–9. [DOI] [PubMed] [Google Scholar]
- Sescousse G, Caldú X, Segura B, Dreher J. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci Biobehav Rev 2013; 37: 681–96. [DOI] [PubMed] [Google Scholar]
- Seymour B, Singer T, Dolan R. The neurobiology of punishment. Nat Rev Neurosci 2007; 8: 300–11. [DOI] [PubMed] [Google Scholar]
- Shinagawa S, Babu A, Sturm V, Shany-Ur T, Toofanian Ross P, Zackey D, et al. Neural basis of motivational approach and withdrawal behaviors in neurodegenerative disease. Brain Behav 2015; 5: e00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron 2003; 39: 701–11. [DOI] [PubMed] [Google Scholar]
- Society for Psychophysiological Research Ad Hoc Committee on Electrodermal Measures. Publication recommendations for electrodermal measurements. Psychophysiology 2012; 49: 1017–34. [DOI] [PubMed] [Google Scholar]
- Sturm VE, Yokoyama JS, Eckart JA, Zakrzewski J, Rosen HJ, Miller BL, et al. Damage to left frontal regulatory circuits produces greater positive emotional reactivity in frontotemporal dementia. Cortex 2015; 64: 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torralva T, Kipps CM, Hodges JR, Clark L, Bekinschtein T, Roca M, et al. The relationship between affective decision-making and theory of mind in the frontal variant of fronto-temporal dementia. Neuropsychologia 2007; 45: 342–9. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Sampson EL, Loy CT, Warren JE, Rossor MN, Fox NC, et al. VBM signatures of abnormal eating behaviours in frontotemporal lobar degeneration. Neuroimage 2007; 35: 207–13. [DOI] [PubMed] [Google Scholar]
- Winston JS, Gottfried JA, Kilner JM, Dolan RJ. Integrated neural representations of odor intensity and affective valence in human amygdala. J Neurosci 2005; 25: 8903–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Gorno-Tempini ML, Seeley WW, Rankin K, Lee SS, Matthews BR, et al. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology 2007; 69: 1424–33. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci USA 1997; 94: 4119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Rev 2003; 41: 88–123. [DOI] [PubMed] [Google Scholar]