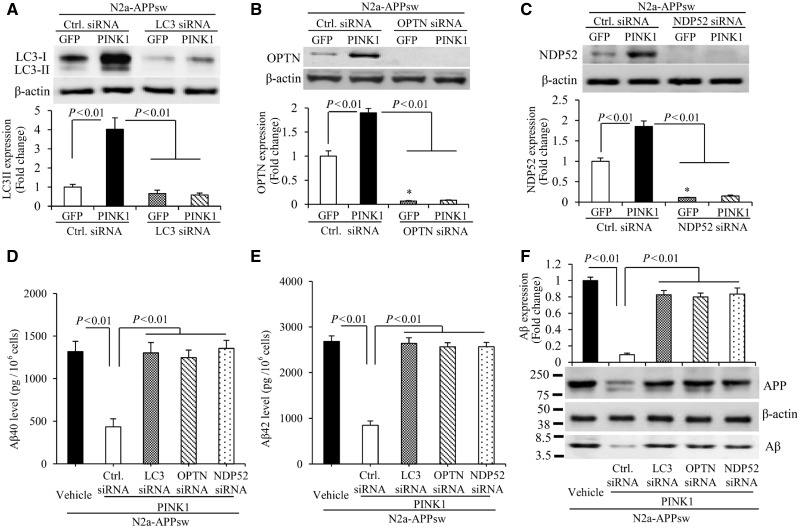
Figure 5.
PINK1 decreases amyloid-β accumulation via activation of autophagy signalling in N2a-APPsw cells. (A–C) Immunoblotting of N2a-APPsw cell lysates for LC3 (A), OPTN (B) and NDP52 (C) in the indicated groups of cells. N2a-APPsw cells were transduced with lentivirus encoding GFP or PINK1 and co-transfected with siRNA against LC3 (A), OPTN (B), NDP52 (C) and control siRNA. The expression levels of LC3-II, OPTN, and NDP52 were eliminated in cells treated with corresponding siRNA compared to control siRNA. Representative immunoblots show the immunoreactive bands for LC3-II (A), OPTN (B), and NDP52 (C), and β-actin served as a loading control. The bar graphs present quantification of immunoreactive bands for LC3-II (A), OPTN (B), and NDP52 (C) normalized to β-actin. *P < 0.01 versus control siRNA treatment group in B and C. n = 3 independent experiments of each group. (D–F) The levels of amyloid-β40 (Aβ40) (D) and amyloid-β42 (Aβ42) (E) in the N2a-APPsw cells transduced with lentivirus encoding GFP or PINK1 and co-transfected with siRNA against LC3, OPTN, NDP52 or control siRNA were measured by amyloid-β ELISA (n = 4). (F) Representative immunoblots show immunoreactive bands for APP and amyloid-β proteins in N2a-APPsw cell lysates with above treatment, and β-actin served as a loading control. The bar graph (F) presents quantification of immunoreactive bands for amyloid-β relative to β-actin. n = 3 independent experiments of each group.