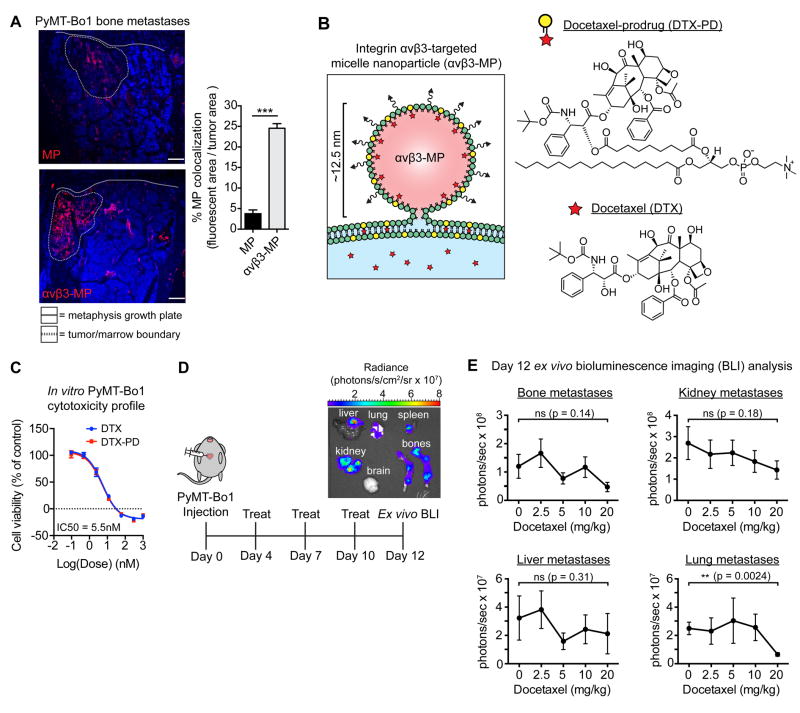
Figure 5.
Micelle nanoparticles targeting integrin αvβ3 colocalize with breast cancer bone metastases and are designed to carry the chemotherapeutic docetaxel. (A) Mice bearing PyMT-Bo1 bone metastases were intravenously injected with rhodamine-labeled (red) non-targeted MPs or αvβ3-MPs. After 3 hours, bones were removed and fresh-frozen sections were immunofluorescently imaged. DAPI nuclear counterstain (blue). Representative images (left), quantification of fluorescent colocalization with the bone metastases, n=3 per group (right). Scale=200 μm. Two-tailed unpaired t-test, *** P<0.001. (B) Schematic of αvβ3-MP-mediated “contact-facilitated drug delivery.” Upon hemifusion, phospholipid-conjugated docetaxel-prodrug (DTX-PD) transfers to the target cell’s plasma membrane, where bioactive DTX is enzymatically liberated by phospholipases and released directly into the cytoplasm. (C) In vitro PyMT-Bo1 cell viability via MTT assay after 72 hours of DTX or DTX-PD treatment. n=2 biological replicates, each in technical triplicate. (D) Treatment schematic of mice intracardiac injected with PyMT-Bo1 cells. Representative ex vivo BLI of tumor-bearing organs on day 12. (E) Following the schematic in Fig. 5D, mice were treated with increasing concentrations of docetaxel, with the cumulative dose as indicated. Day 12 ex vivo PyMT-Bo1 metastatic tumor burden by BLI analysis, n=6. Bone, Kidney, Lung: two-tailed unpaired t-test. Liver: two-tailed Mann–Whitney U-test. All with Bonferroni correction for a priori multiple comparisons between control 0 mg/kg and each DTX treatment group, ** P<(0.01/4), * P<(0.05/4), ns=(not significant). Data presented as mean±SEM.