Figure 3.
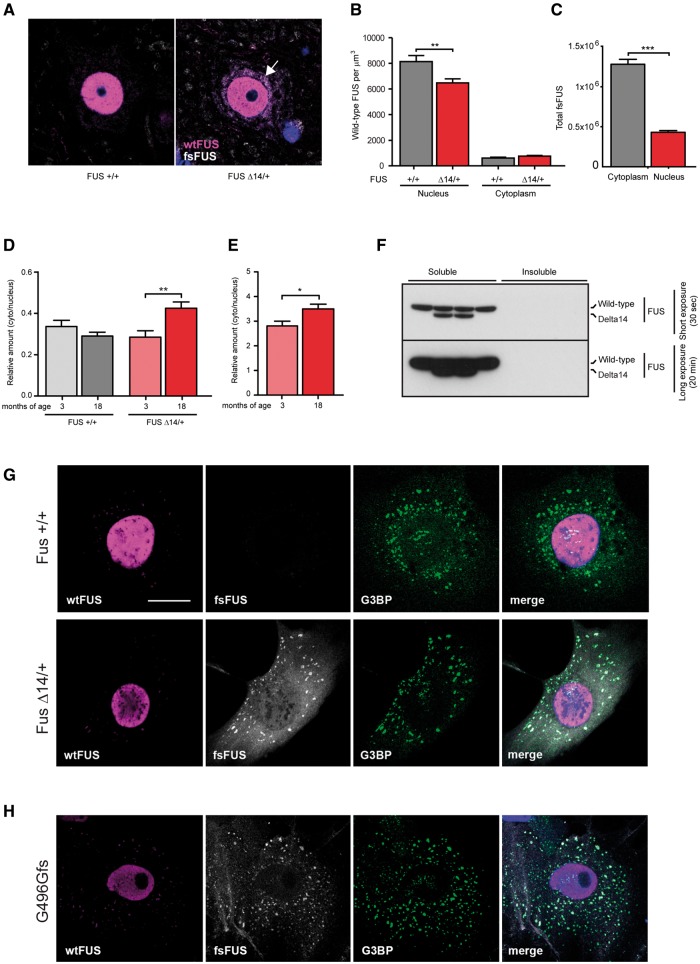
FUS Delta14 protein mislocalizes to the cytoplasm but does not aggregate. (A) Using a C-terminal FUS antibody that detects only wild-type FUS (magenta) and our novel mutant-specific FUS antibody (fsFUS, white) to quantify the distribution of FUS protein in lumbar motor neurons. Perinuclear accumulations of fsFUS are observed (arrow). (B) Quantification of distribution of wild-type FUS protein in the nucleus versus cytoplasm of motor neurons in wild-type versus heterozygous FUS Delta14 mice. Wild-type FUS is located almost exclusively in the nucleus in both wild-type and FUS Delta14 mice, with significantly less nuclear wild-type FUS in FUS Delta14 motor neurons (P = 0.0049). (C) Quantification of distribution of FUS Delta14 protein in the nucleus versus cytoplasm of motor neurons in heterozygous FUS Delta14 mice. There is significantly more cytoplasmic FUS Delta14 protein compared to nuclear protein, although 25% of FUS Delta14 protein is nuclear in spite of the lack of the nuclear localization signal (P < 0.0001). The distribution of FUS changes with age in Delta14 mice. (D) No differences in the cytoplasmic:nuclear ratio were observed between 3 and 18 months of age for the distribution of wild-type FUS protein in wild-type mice. However, there was a significant increase in the cytoplasmic:nuclear ratio of wild-type FUS protein in heterozygous FUS Delta14 mice (P = 0.0062). (E) There is an increase in cytoplasmic:nuclear ratio of mutant FUS protein at 18 months of age in heterozygous FUS Delta14 mice (P = 0.0486). (F) There is no insoluble FUS protein present in the lumbar spinal cord of 12-month-old wild-type and heterozygous FUS Delta14 mice. SOD1 G93A spinal cord was used as a positive control (data not shown). Mutant FUS protein is preferentially recruited into stress granules. (G) In wild-type and heterozygous adult mouse fibroblasts almost no wild-type FUS protein (wtFUS, magenta) is detected in cytoplasmic stress granules. However, in heterozygous FUS Delta14 fibroblasts a significant proportion of cytoplasmic frameshift FUS protein (fsFUS, white) co-localizes with the stress granule marker G3BP, green. (H) Human fibroblasts with a frameshift mutation in FUS (G496Gfs), which have the same frameshift peptide sequence at the C-terminus that is recognized by our novel frameshift FUS antibody (fsFUS, white), show the same recruitment of cytoplasmic frameshift FUS protein to stress granules (G3BP, green) as observed in FUS Delta14 adult mouse fibroblasts. Recruitment of wild-type FUS protein to stress granules is limited (wtFUS, magenta). Scale bar = 20 µm.