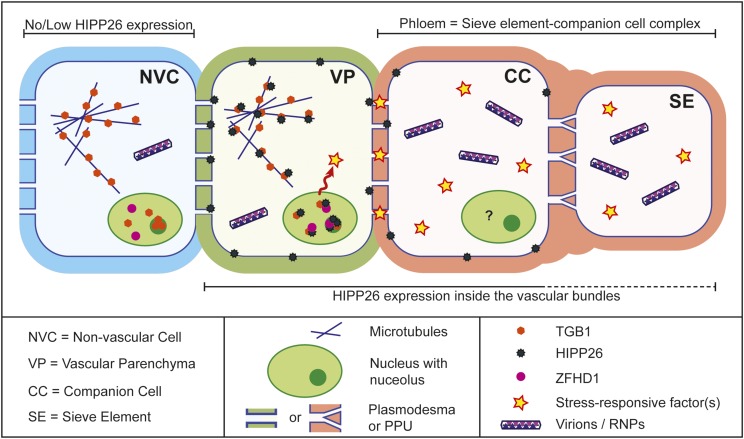
Figure 10.
Model of the mechanism of long-distance movement. In a PMTV infection, TGB1 localizes to microtubules and traffics to the nucleus, where it is localized in the nucleoplasm and nucleolus (as shown in the nonvascular cell [NVC]; blue). HIPP26 is expressed mainly inside the vascular bundle, predominantly in regions of the bundle containing phloem cells (phloem parenchyma, companion cells, and sieve elements), and also in other vascular parenchyma cells, which can sometimes be identified as xylem parenchyma due to their proximity to metaxylem elements. Once the virus reaches a vascular bundle where HIPP26 is expressed, the TGB1 binds near the CVVM (prenyl domain) of NbHIPP26 at the plasma membrane, most likely in the vicinity of PD. This de-S-acylates HIPP26, which releases the NbHIPP26/TGB1 complex from the plasma membrane into the cytosol, from where it localizes to microtubules and subsequently moves via importin-α-directed transport to the nucleus and nucleolus, where HIPP26 interacts with the transcriptional activator ZFHD1. Interaction with ZFHD1 stimulates host proteins and/or stress response factors such as NAC transcription factors, along with molecules that increase the size exclusion limit of the PD at the vascular parenchyma (VP)-companion cell (CC) interface. These PDs are seen as a key control point for entry into the sieve element (SE)/companion cell complex and an important barrier to systemic spread for viruses. The pore-plasmodesma units (PPU) that connect companion cells and sieve elements are known to have a large size exclusion limit, so the sieve element/companion cell complex has been proposed to be a single domain where even large molecules can move relatively freely into the sieve element. It is difficult to differentiate between what may occur in the phloem parenchyma and companion cells; protein expression and interactions may be different or the same in these cell types, indicated by the question mark in the companion cell nucleus. The key point is that we believe the virus exploits the systemic signaling response to drought in order to gain entry into the phloem. Once inside the phloem tissue, in the presence of host proteins that gate PD, virions or RNPs can enter the sieve element for systemic spread.