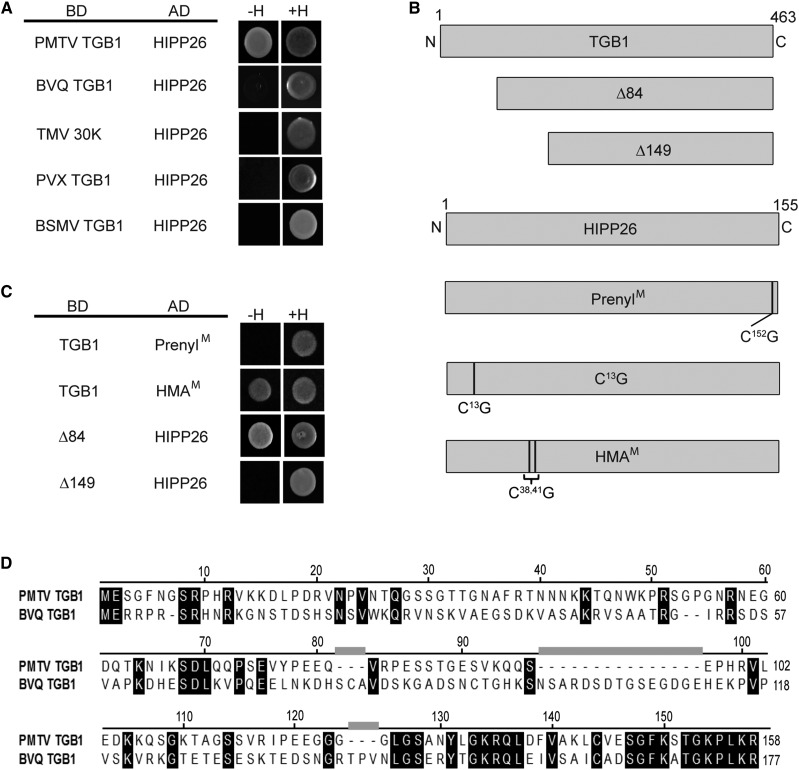
Figure 2.
Y2H interaction analysis. A, Virus movement proteins, PMTV TGB1, beet virus Q (BVQ) TGB1, TMV 30K, potato virus X (PVX) TGB1, and barley stripe mosaic virus (BSMV) TGB1 proteins were fused to the LexA binding domain (BD) and tested for interaction with HIPP26 fused to the p6 activation domain (AD) on double dropout (+H) or triple dropout (−H) medium. B, Diagrammatic representation of the wild type and mutants in PMTV TGB1 (∆84 and ∆149 deleted by amino acids 1–84 and 1–149, respectively) and the wild type and mutants of HIPP26 showing the positions of the Cys residues changed to Gly in putative prenylation (PrenylM), S-acylation (C13G), and heavy metal-associated (HMAM) domains. C, TGB1 wild type and deletion mutants were tested for interaction with HIPP26, PrenylM, and HMAM. D, Amino acid sequence alignment of PMTV and BVQ N-terminal amino acids.