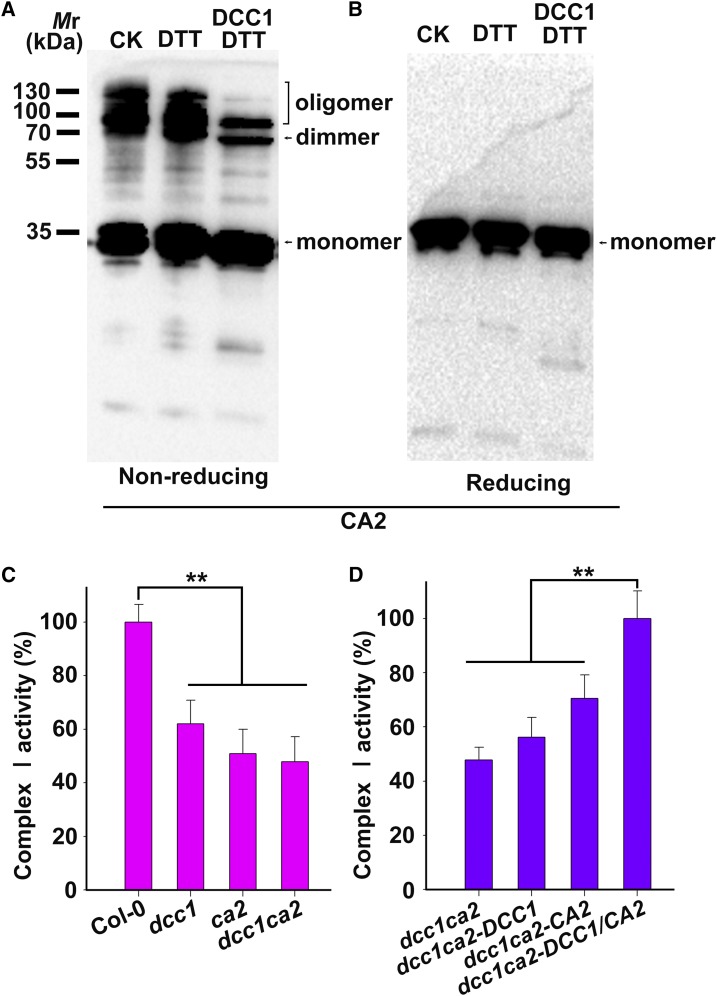
Figure 5.
DCC1 regulates Complex I activity via CA2 protein. A and B, CA2 dimer and oligomer were reduced by DCC1. Purified CA2-His protein was incubated in the assay mixture with or without DCC1 GST-cleaved protein (5 μm) for 30 min at room temperature and then subjected to nonreducing (A) or reducing (B) SDS-PAGE followed by immunoblot analysis. DTT (0.3 mm) was added to recycle DCC1 Trx activity. The positions of the monomer, dimer, and oligomer of CA2 are indicated. CA2 dimer and oligomer showed significantly reduced levels when incubated with DCC1 proteins (right lane). The reaction mixture without DCC1 proteins was used as the control (left and middle lanes). CK, Control check. C, Relative activity of mitochondrial respiratory Complex I. Mitochondria were isolated from calli (0.1 g) of wild-type Col-0, dcc1, ca2, and dcc1ca2 cultured on SIM at 16 d. Mitochondrial proteins (20 μg) were used to determine the Complex I activity. The absorbance of all samples was measured at 340 nm for 60 min using a plate reader. Complex I activity was calculated from the decrease in absorbance per minute. Functional loss of DCC1 or CA2 resulted in reduced activity of Complex I. D, Relative activity of Complex I in calli of dcc1ca2 after the addition of recombinant CA2 or DCC1 protein. Purified CA2-His proteins and DCC1-His proteins were added to the assay mixture. Only the addition of both DCC1 and CA2 proteins completely rescued the reduced Complex I activity. Asterisks indicate significant differences: **, P < 0.01 (Student’s one-tailed t test).