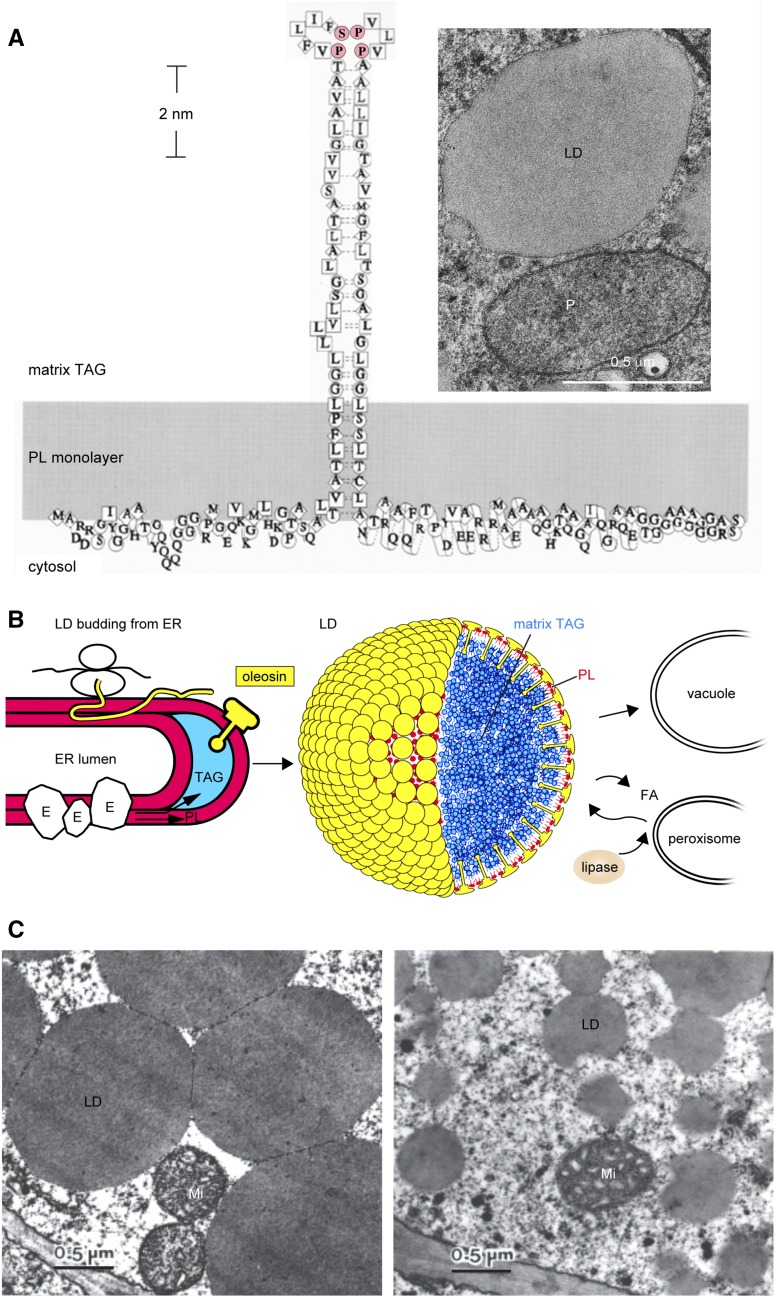
Figure 1.
Seed LDs and oleosin. A, Model of an oleosin molecule on the surface of an LD (Tzen and Huang, 1992). The shaded area represents a monolayer of PLs with the head groups facing the cytosol. Symbols of amino acids and their hydropathy indices are as follows: square for very hydrophobic (4.5–3.8), diamond for hydrophobic (2.8–1.8), circle for amphipathic (−0.4 to −1.6), and no enclosure for hydrophilic (−3.2 to −4.5) residues. The N-terminal segment is shown without any well-defined structure. The central hydrophobic segment is depicted as a hairpin with a Pro knot at the loop (P and S in pink); its secondary structure is unknown. The C-terminal segment is an amphipathic α-helical structure interacting with the PL surface and a variable-length nonconserved peptide at the terminus. The monolayer of PL enclosing an LD is revealed in the inset electron micrograph of a shoot apex cell of a 1-d-old maize seedling (courtesy of Richard Trelease). LD, peroxisome (P), and plastid (at the top right corner; not labeled) are enclosed with one, two, and four electron-dense lines, representing one (one half-unit membrane), two (one unit membrane), and four (double membrane) PL layers, respectively. B, Model of LD synthesis during seed maturation and degradation during seed germination. Oleosin is shaped like a mushroom, which includes the mushroom head (N- and C-terminal amphipathic segments) and a stalk (the central hydrophobic hairpin; courtesy of Ariel Kuan [Tzen and Huang, 1992]). Left, Budding LD on rough ER during seed maturation. The overall structure includes the ER lumen, two PL layers (red), sequestered TAGs (blue), a ribosome with an mRNA synthesizing an oleosin polypeptide (thin yellow ribbon, of unknown configuration), and ER enzymes (irregular spheres [E]) for the synthesis of TAGs and PLs. All constituents are not drawn to scale. Middle, LD model, with oleosin (yellow) and PLs (red) enclosing the matrix TAGs (blue). All three types of molecules are drawn approximately to scale, but the diameter of the LD has been reduced 24 times to reveal clearly the surface structure. Right, LD degradation during germination. Lipase associated with core retromer binds to peroxisome, which transfers the lipase to LD for lipolysis; the enzymic product fatty acid (FA) is transferred to peroxisome for gluconeogenesis. A TAG-depleted ghost may join with the vacuole membrane. Alternatively, the whole LD is engulfed by a vacuole. All constituents are not drawn to scale. C, Electron micrographs of LDs in embryos of two maize lines with diverse TAG-oleosin ratios. LDs in Illinois High Oil line (with a high ratio of TAGs to oleosin) are larger and spherical. LDs in Illinois Low Oil line (with one-seventh the TAG-oleosin ratio) are smaller and have an irregularly contoured surface. Double-membraned mitochondria (Mi) are present. LDs isolated from the two lines are stable without coalescence or aggregation and maintain their respective sizes and shapes (Ting et al., 1996).