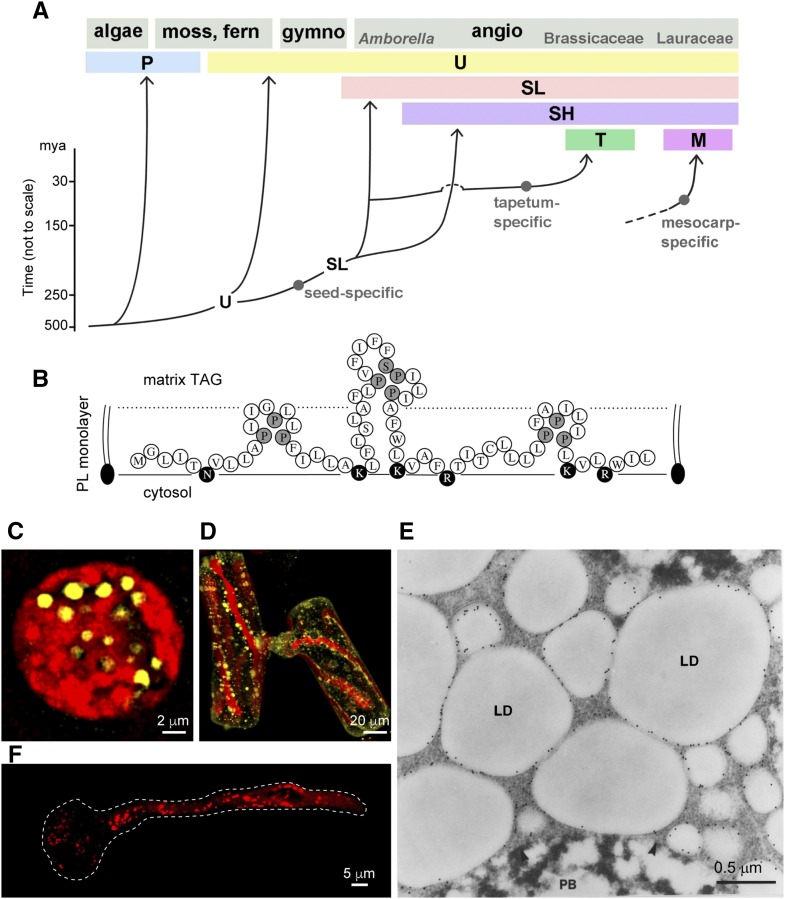
Figure 2.
Evolution of the six oleosin lineages and LDs housing some of these oleosins. A, Cartoon of the evolution of six oleosin lineages: P (primitive), U (universal), SL (seed low molecular weight), SH (seed high molecular weight), T (tapetum in Brassicaceae), and M (mesocarp in Lauraceae). The origin of M oleosin is unknown and is shown with a broken line. (Modified from Huang and Huang [2015].) B, Predicted polypeptide organization of an oleosin-like protein on the surface of an LD in the primitive green algae C. reinhardtii and V. carteri. The polypeptide has one hydrophobic hairpin with a loop identical to that of oleosin and two adjacent hairpins with less similar loops. Pivotal Pro and Ser residues are shaded, and hydrophilic residues are darkened. All three hairpins are shorter than the oleosin hairpin (for comparison, see Fig. 1A). The cytosol, the surface PL monolayer, and the matrix TAGs are labeled. This oleosin-like protein could be the precursor of oleosin or a remnant of degenerating oleosin (Huang et al., 2013b). C and D, P oleosin-containing LDs in C. reinhardtii zygote and conjugating S. grevilleana cells viewed by CLSM. The cells were stained for LDs with BODIPY (shown in yellow) in the presence of chloroplasts (autofluorescence [in red]; Huang et al., 2013b). E, SL and SH oleosin-containing LDs in maize embryo of seed (after 12 h of soaking) subjected to immunogold labeling with antibodies against the maize oleosin, viewed by electron microscopy. LD and protein body (PB) are visible (Fernandez et al., 1988). F, U oleosin-containing LDs in Arabidopsis pollen after germination. The pollen grain produces a tube that carries the two sperm nuclei to the ovary. LDs travel to the tube tip via streaming cytoskeleton. LDs were stained with Nile Red (shown in red). The boundary of the pollen grain and tube is shown with a dotted white line. (Courtesy of Ming-Der Huang.)