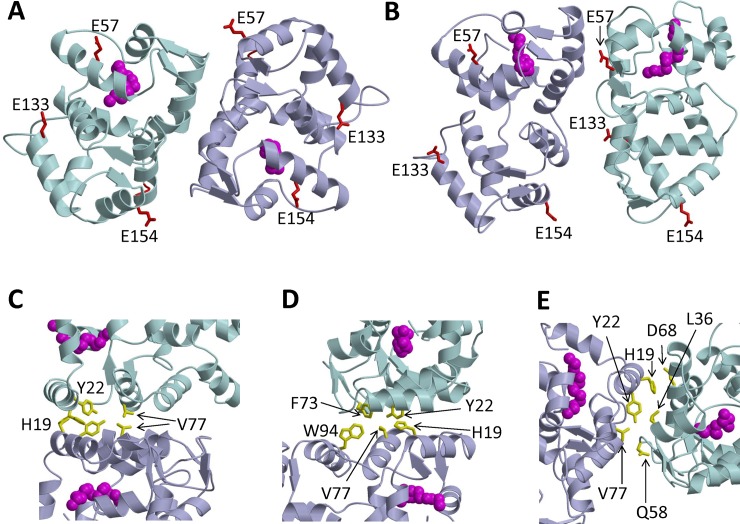
Fig 5. Structural model of GCAP1 dimer.
Ribbon diagram of main chain structures of the GCAP1 symmetric dimer (A) and asymmetric dimer (B) that are both consistent with DEER intermolecular distances for spin-label attached to E57C, E133C and E154C. Exposed residues mutated to Cys for DEER studies (E57, E133 and E154) are colored red in panels A and B. Myristoyl group is highlighted magenta. (C) Close-up view of the symmetric dimer showing hydrophobic residues (side-chains of H19, Y22, and V77 colored yellow) at the dimer interface. (D) Close-up view of the symmetric dimer showing intermolecular hydrophobic contacts between Y22, F73, V77 and W94. (E) Close-up view of the asymmetric dimer showing hydrophobic residues (H19, Y22, and V77 colored yellow) at the dimer interface.