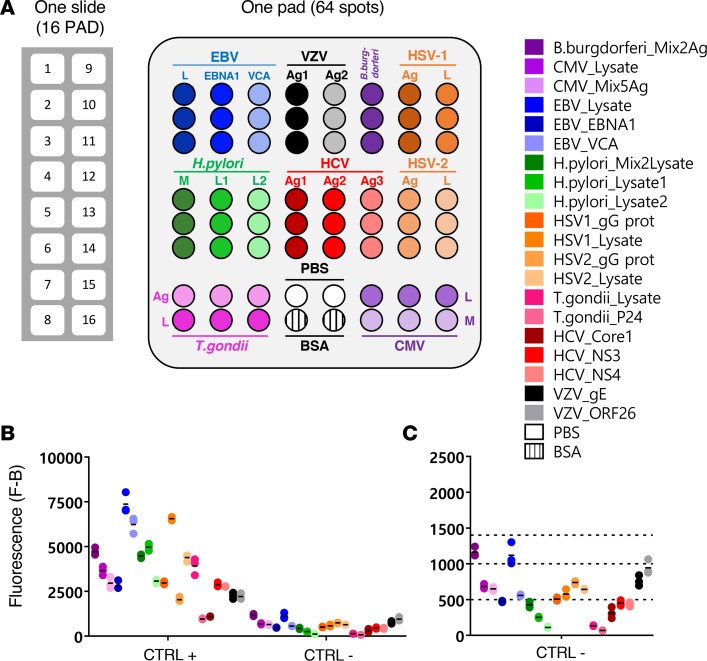
Figure 1. Description of the MIAA assay.
(A) The multiplex infectious-antigen array (MIAA) assay consists of microarray slides that contain 16 identical pads; 1 pad (represented enlarged) is shown in detail, with Ag or lysate spotted in triplicate. For each patient, serum and purified monoclonal (mc) IgGs are examined in parallel using the MIAA assay; each type of sample is tested in duplicate or more (some samples were tested 6 times within 1 experiment). Fluorescence signal is used to determine the serological status of each sample (15). (B) Human serum samples containing polyclonal IgGs specific for each of the 9 infectious pathogens (= positive controls, CTRL+) were used to set up the assay and assess reproducibility. Human serum control samples that did not contain IgG specific for 1 or several pathogens (= negative controls, CTRL–) served to evaluate nonspecific binding and to determine the fluorescence threshold of specific positivity for each pathogen, Ag, or lysate (L), or mix of Ag (M). Shown is the net fluorescence intensity of the samples after subtracting background fluorescence (F–B). (C) Detail of negative controls and thresholds of positivity. Three fluorescence thresholds of specific positivity were used in all MIAA experiments: 500 for hepatitis C virus (HCV), H. pylori, and T. gondii; 1,000 for CMV, herpes simplex virus-1 (HSV-1), and HSV-2; and 1,400 for EBV, varicella zoster virus (VZV), and B. burgdorferi. Signals below these thresholds were considered to be negative. Dots may be superimposed; black horizontal bars represent the means of results obtained for a pathogen, Ag, or lysate. Positive and negative controls are run in every MIAA experiment, as internal controls.