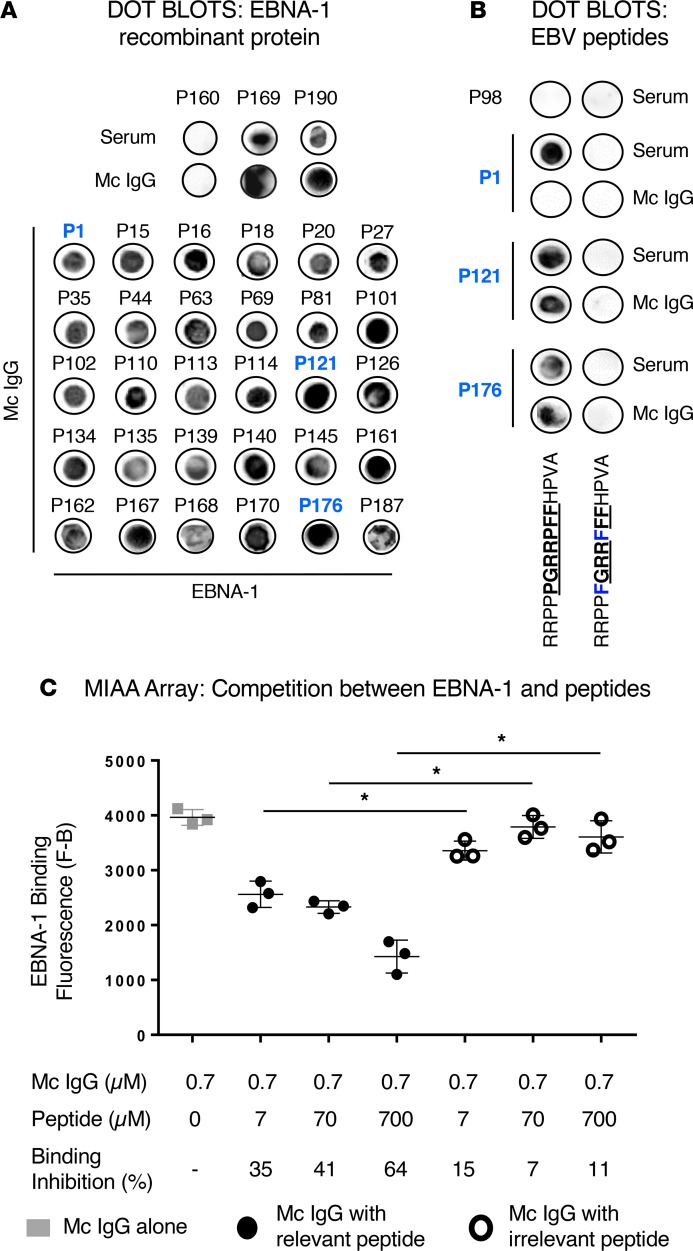
Figure 10. Confirmation of the specificity of EBNA-1 recognition of purified mc IgGs.
(A) Dot blotting assays with purified recombinant EBV nuclear antigen-1 (EBNA-1) were first performed in parallel with serum and purified monoclonal (mc) IgG from 3 patients: P169 and P190, patients with EBNA-1–specific mc IgG as assessed by the multiplex infectious-antigen array (MIAA) assay, as positive controls; and patient P160, whose mc IgG did not recognize EBNA-1 as assessed by the MIAA assay, as a negative control. EBNA-1 dot blotting was then performed with the purified mc IgGs of 30 additional patients with EBNA-1–specific mc IgG as assessed by the MIAA assay. (B) Dot blotting assays performed by coating relevant (RRPPPGRRPFFHPVA) or irrelevant (RRPPFGRRFFFHPVA) EBNA-1–derived peptides and studying reactivity with serum and purified mc IgGs. Amino acids of the relevant epitope are in bold and underlined. The modified amino acids in the irrelevant peptide are shown in blue. Signal was obtained only with relevant peptide, but only 2 of 24 of the purified mc IgGs that could be tested bound to the relevant peptide. The serum of patient P98 does not contain IgG specific for EBNA-1. Patient P1 is shown as an example of serum containing polyclonal IgGs that bound to the relevant peptide, while the purified mc IgG did not bind. (C) Dose-dependant inhibition of recognition of EBNA-1 protein by purified mc IgG specific for EBNA-1 in the presence of the relevant peptide in the MIAA assay. Recombinant EBNA-1 protein was spotted on the array. A purified mc IgG found to be EBNA-1 specific with the MIAA assay and to recognize the relevant EBNA-1–derived peptide RRPPPGRRPFFHPVA with the dot blot assay, was preincubated with different concentrations of this peptide for 1 hour at room temperature before adding the mc IgG to the MIAA pad. The irrelevant peptide RRPPFGRRFFFHPVA was used as a negative control. Results are means ± SD of triplicates. *P < 0.05 by ANOVA. Experiments were performed at least twice.