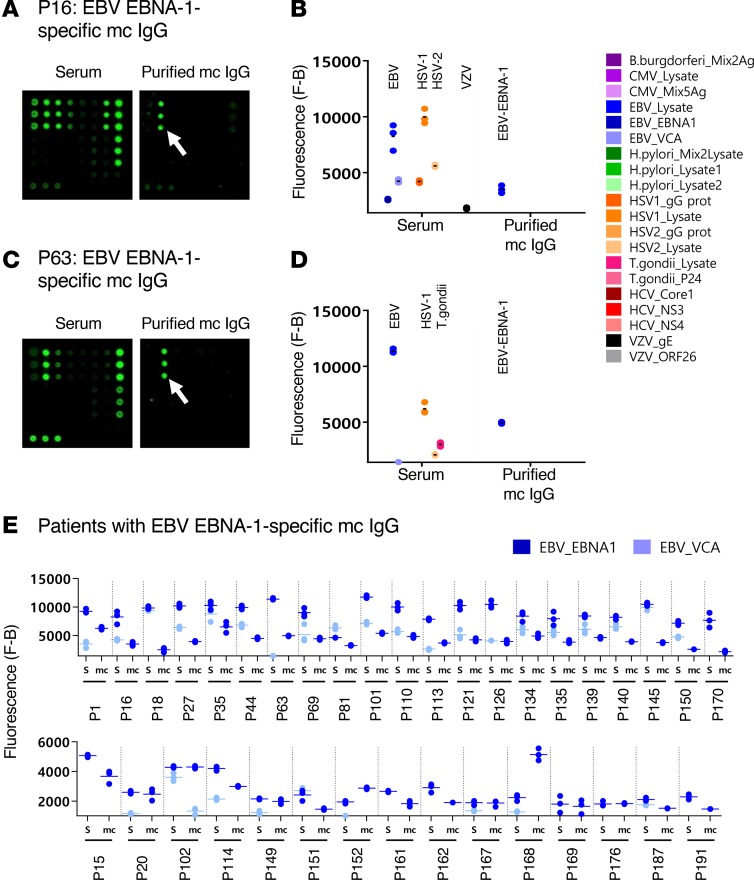
Figure 3. EBV-specific mc IgG as determined by the MIAA assay.
For each patient, serum and purified monoclonal (mc) IgGs were incubated in parallel in the multiplex infectious-antigen array (MIAA) assay; results of hybridization are shown for patients P16 (A and B) and P63 (C and D). (A and B) For P16, serum contained IgG that recognized EBV nuclear antigen-1 (EBNA-1), EBV viral capsid antigen (VCA), herpes simplex virus-1 (HSV-1), HSV-2, and varicella zoster virus (VZV) glycoprotein E (gE), whereas the purified mc IgG recognized EBNA-1 only. (C and D) For P63, serum contained IgG that recognized EBNA-1, EBV VCA, HSV-1, HSV-2, and T. gondii, whereas the purified mc IgG recognized EBNA-1 only. (B and D) Quantification of the fluorescence signals generated by the serum and purified mc IgG of patients P16 (B) and P63 (D) as assessed with the MIAA assay. (E) Results obtained for the 36 patients found to have an EBNA-1–specific mc IgG. For each patient, the results obtained for EBV with the patient’s serum (S) and purified mc IgG are shown in dark blue (EBNA-1) or light blue (VCA). The fluorescence values shown for EBNA-1 or EBV VCA were obtained after subtraction of the threshold of specific positivity for each pathogen, protein, or lysate (1,400 for EBV). Dots may be superimposed; horizontal bars represent the means of results obtained for a pathogen, Ag, or lysate. Experiments were performed at least twice.