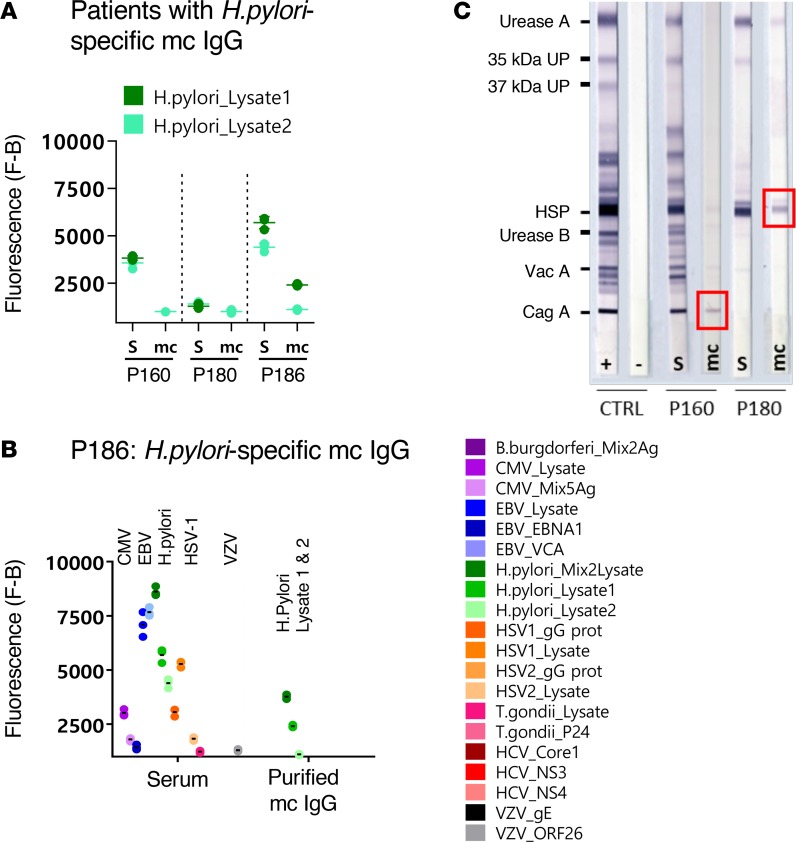
Figure 8. H.pylori–specific mc IgGs as determined by the MIAA assay.
(A) Results of the multiplex infectious-antigen array (MIAA) assay for the 3 patients with a H. pylori–specific monoclonal (mc) IgG. For each patient, the results obtained for H. pylori lysate 1 and lysate 2 with the patient’s serum (S) and purified mc IgG are shown. (B) The results obtained for patient P186 are detailed: serum P186 contained IgG that recognized CMV, EBV nuclear antigen-1 (EBNA-1), EBV viral capsid protein (VCA), H. pylori, herpes simplex virus-1 (HSV-1), HSV-2, T. gondii, and varicella zoster virus (VZV) ORF-26, whereas the purified mc IgG recognized only the H. pylori lysates (dark green: mix of lysates 1 and 2; medium green: lysate 1; light green: lysate 2). Note that certain H. pylori proteins and Ags are likely present both in lysate 1 and lysate 2. The fluorescence values shown for each pathogen, Ag, or lysate were obtained after subtraction of the threshold of specific positivity of the pathogen, Ag, or lysate (500 for H. pylori). Dots may be superimposed; horizontal bars represent the mean of results obtained for a pathogen, Ag, or lysate. Experiments were performed at least twice. (C) The immunoblot assay was performed using the commercial kit Helico Blot 2.1 (MP Biomedicals), which consisted of a Western blot made from bacterial lysate of H. pylori strain ATCC 49503. The test strip contained H. pylori Ags with molecular weights of 116 kDa (CagA), 89 kDa (VacA), 65 kDa (urease B), 60 kDa (heat shock protein [HSP]), 37 kDa (H. pylori undetermined protein [UP]), 35 kDa (H. pylori UP), and 30 kDa (urease A) as separate bands. The assay was performed and interpreted according to the instructions of the manufacturers. Experiments were performed at least twice.