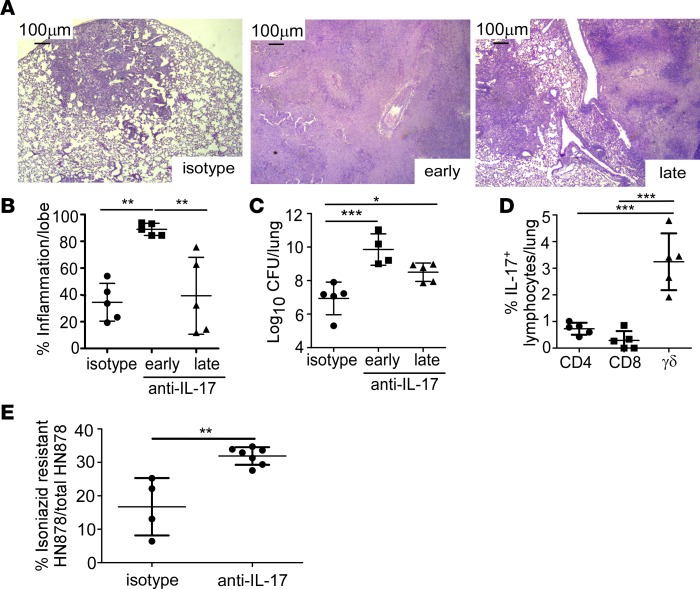
Figure 2. Early IL-17 neutralization results in hypoxic granuloma formation and loss of Mtb control.
FeJ mice were aerosol infected with approximately 100 CFU Mycobacterium tuberculosis (Mtb) clinical strain HN878. IL-17 was neutralized (A–E) early (10–24 days postinfection [d.p.i]) or (A–D) late (24–31 d.p.i) and mice were harvested at 37 d.p.i. (A) Representative images from formalin-fixed, paraffin-embedded (FFPE) lung sections stained using H&E, ×50 magnification. (B) Lung inflammation in H&E–stained FFPE sections was quantified using the morphometric tool of the Zeiss Axioplan microscope (n = 5 mice/group). (C) Lung bacterial burden was assessed by plating lung homogenates of both isotype-antibody-treated (n = 5 mice) and IL-17–neutralized mice (n = 4 mice/early anti–IL-17; n = 5 mice/late anti–IL-17). (D) Lungs were processed to single-cell suspensions and flow cytometry was used to determine the lymphocyte populations producing IL-17 in the lungs of HN878-infected FeJ mice (n = 5 mice/group). (E) At 37 d.p.i., bacterial burden was plated and the percentage of isoniazid-resistant HN878 was measured by normalizing growth on isoniazid plates to total HN878 growth on non-isoniazid plates (n = 4 mice/isotype, n = 7 mice/anti–IL-17). All data shown as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 by 1-way ANOVA (B–D) or Student’s t test (E).