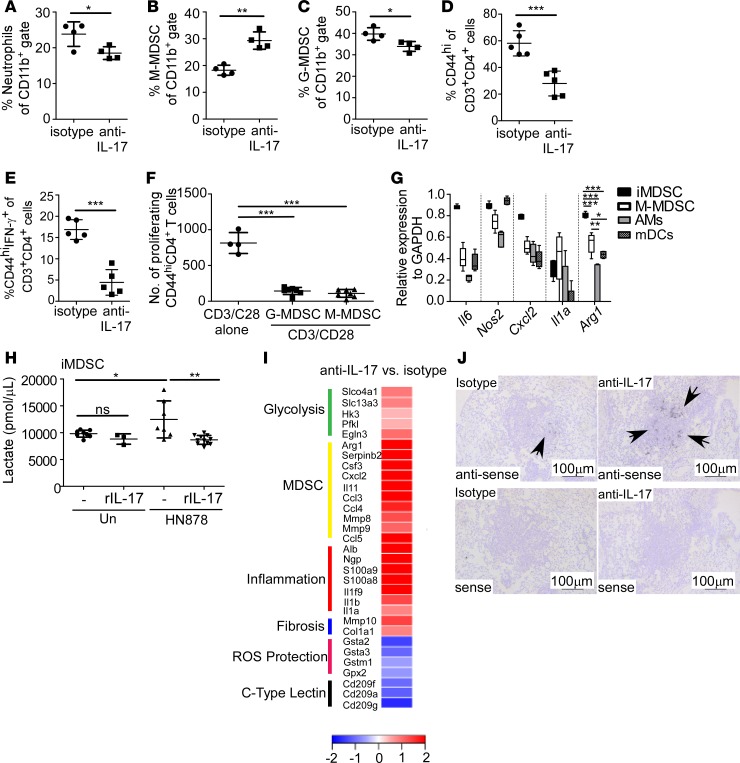
Figure 3. IL-17 limits accumulation of monocytic MDSCs and shifts towards aerobic glycolysis.
FeJ mice were aerosol infected with approximately 100 CFU Mycobacterium tuberculosis clinical strain HN878 and treated with isotype or anti–IL-17 antibody as described in Figure 1. At 30 days postinfection (d.p.i.), lungs were processed to a single-cell suspension, and flow cytometry was used to assess frequencies of (A) neutrophils (n = 4 mice/group), (B) monocytic myeloid-derived suppressor cells (M-MDSCs; n = 4 mice/group), (C) granulocytic MDSCs (G-MDSCs n = 4 mice/group), (D) activated CD44hiCD4+ T cells (n = 5 mice/group), and (E) activated CD44hiCD4+ IFN-γ–producing T cells (n = 5 mice/group) in the lungs. (F) FeJ mice were infected with approximately 100 CFU HN878 and treated with anti–IL-17. At 37 d.p.i., lung G-MDSCs and M-MDSCs were sorted based on the gating strategy shown in Supplemental Figure 2C and cocultured with anti–CD3/CD28–stimulated, CFSE-stained CD4+ T cells for 3 days, and the number of proliferating CD44hiCD4+ T cells normalized to 10,000 cells are shown (n = 4 biological replicates/anti–CD3/CD28 alone, n = 7 mice/G-MDSC or M-MDSC + anti–CD3/CD28). (G) qRT-PCR was used to determine expression of Nos2, Il6, Il1a, Arg1, and Cxcl2 in FACS-sorted M-MDSCs, alveolar macrophages (AMs), and myeloid DCs (mDCs) from HN878-infected mice (n = 5 mice) at 31 d.p.i., or HN878-infected in vivo–generated MDSCs (iMDSCs; n = 5 biological replicates) from FeJ bone marrow. (H) Lactate accumulation in culture supernatants of uninfected (n = 8 biological replicates), uninfected and rIL-17–treated (100 ng/ml, n = 3 biological replicates), untreated HN878-infected (MOI 0.1) (n = 8 biological replicates), and HN878-infected rIL-17–treated (n = 11 biological replicates) iMDSCs was assessed using a lactate assay. (I) RNA-Seq analysis depicting fold change of specific gene expression in lungs of anti–IL-17–treated HN878-infected FeJ mice (n = 5 mice) over the expression in isotype-treated HN878-infected mice (n = 3 mice) at 30 d.p.i. (J) Cxcl2 mRNA localization in lung was determined by in situ hybridization. Representative images are shown, ×100 magnification; arrows depict Cxcl2 expression within granulomas. All data shown as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001 by Student’s t test (A–E), or 1-way ANOVA (F–H). ns, not significant.