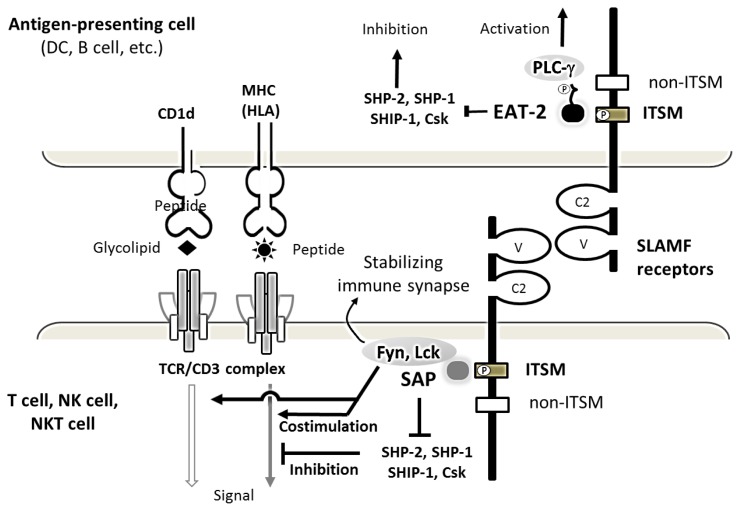
Figure 2. Structure and function of SLAMF receptor in an immune synapse.
The SLAMF receptors are structurally characterized by IgV and IgC2 domains within an extracellular portion and one or more ITSMs, depicted as a closed rectangle, within the cytoplasmic portion. The mostly homophilic interactions between SLAMF receptors result in their costimulatory effects on TCR/CD3 complex signaling pathway. When the SLAMF receptor is engaged by its ligand, cytoplasmic domain ITSMs with tyrosine-based motifs undergo phosphorylation, recruiting adaptors proteins, SAP or EAT-2. SAP can then recruit the Src family protein tyrosine kinase Fyn or Lck, which is important for activation via SLAM family receptors. The coupling of EAT-2 carboxyl-terminal tail to the PLC-γ SH2 domains leads to an additional activation pathway. ITSM-like motif (non-ITSM) depicted as an unfilled rectangle does not bind SAP or EAT-2. SAP is mostly expressed in T cells, while EAT-2 is primarily expressed in antigen-presenting cells.