Abstract
Rationale:
Blue rubber bleb nevus syndrome (BRBNS) is a rare disease characterized by multiple venous malformations. The gastrointestinal bleeding and secondary iron deficiency anemia are the most common complications. There are currently no effective treatments for BRBNS. Here, we report a case of successful treatment with a small dose of sirolimus of a BRBN patient with a de novo gene mutation.
Patient concerns:
A 12-year-old female was admitted to our hospital with multiple hemangiomas for 12 years. The patient often displayed melena; she recently received transfusion of 2 units of red blood cells once every 2 weeks. Multiple fist-sized hemangiomas were piled up on both sides and back of the neck, and were also noted on the arms, legs, chest, back, and on the tip of the tongue. The laboratory findings demonstrated severe anemia. Blood sample sequencing detected a heterozygous de novo mutation c.2545C > Tin the TEK gene.
Diagnoses:
Based on these findings, final diagnosis of Blue rubber bleb nevus syndrome (BRBNS) was made.
Interventions:
After the diagnosis, low-dose sirolimus was orally administered.
Outcomes:
The patient's hemoglobin was increased after treatment with sirolimus for 1 month. Since the initial treatment with sirolimus, she had not received any blood transfusions. The skin and mucosal hemangioma decreased significantly, and new digestive tract hemorrhage, muscle hematoma, or adverse drug reactions were not observed.
Lessons:
we report a case of a mutation in exon 15 of the TEK gene leading to BRBN. It was successfully treated with a small dose of sirolimus as an alternative to blood transfusion in order to save the of BRBN patient's life.
Keywords: Blue rubber bleb nevus syndrome, sirolimus, TEK gene
1. Introduction
Blue rubber bleb nevus syndrome (BRBNS) is a rare disease characterized by multiple venous malformations caused by the vascular development dysplasia, which is related to genetic abnormalities. BRBNS was first described by Gascoyne in 1860; however, it was not fully characterized until 1958 by William Bean, for which the disease has been termed “Bean syndrome.” Hitherto, more than 269 cases of BRBNS have been reported in the literature,[1] and 12 patients have been previously reported in China.[2]
The lesions most frequently occur on the skin and in the gastrointestinal system but may also involve visceral organs. The cutaneous lesions are often found on the neck and limbs, and the lesions of the visceral organs are distributed orbitally. They are also found in the central nervous system, skeleton, and all over the body.[2–4]
The lesions of cutaneous manifestation are dark blue in color, soft, rubbery blebs, which are readily compressible, refilling slowly upon release of pressure. The gastrointestinal bleeding and secondary iron deficiency anemia are the most common symptoms. A majority of the patients need adjuvant treatment such as blood transfusion, complementing the iron agent, and also surgery in order to remove the aberrant large blood vessels.
Till date, the pathogenesis of the BRBNS is uncertain. Although BRBNS has been identified as autosomal familial inheritance and associated with chromosome 9p, a majority are sporadic cases.[2,5] Activating mutations in the angiopoietin receptor TIE2/TEK have been determined as the cause for BRBNS.[6]
Hyperactive mTOR signaling is an underlying mechanism for the abnormal angiogenesis associated with tumorigenesis.[7] Sirolimus inhibits the mTOR pathway and prevents proliferation and protein synthesis in many types of mammalian cells.[8]
Herein, we report a case with BRBNS in a 12-year-old female. We identified the anomalies of exon 15 in TEK, and Next-Gen sequencing revealed a de novo mutation (c.2545C > T, Arg849Trp). We used a low dose of sirolimus as an antiangiogenesis agent. After 1-year treatment, hemoglobin increased steadily; skin and mucosal hemangioma decreased significantly; and no new digestive tract hemorrhage, muscle hematoma, or adverse drug reactions were observed.
2. Case presentation
2.1. Clinical course and diagnosis
A 12-year-old female was admitted to our hospital with multiple hemangiomas for 12 years. According to the foster parent's description, multiple cutaneous hemangiomas were observed upon birth, which had increased in size and number over time. The patient often displayed melena, multiple hemangiomas mainly on the head and neck, limbs and trunk, as well as, those appearing on the tip of the tongue increased gradually. Before admission to our hospital, her previous electrocardiogram, thoracoabdominal CT, and electroencephalogram (EEG) showed abnormal results. Also, hemangiomas were surgically removed from her left leg and back; although a detailed information about the surgery was not clear. One year ago, she underwent gastrointestinal endoscopy, and multiple hemangiomas were found in the digestive tract, but no specific treatment was administered. She recently received transfusion of 2 units of red blood cells once every 2 weeks.
Physical examination: Blood pressure 112/69 mm Hg, weight 35.6 kg, and nutritional status were within the normal range. Multiple hemangiomas were observed on both sides and back of the neck, which were fist-sized and in heaps. A mixture of small and large hemangiomas was also noted on the arms, legs, chest, back, and on the tip of the tongue. The hemangioma is dark blue in color, soft, rubbery, and compressible on palpation. The left leg showed 10-cm-long surgery scars. Grade 4/6 similar blowing systolic murmurs in zones of each heart valve auscultation were heard, and the rest of the physical examination did not reveal any abnormalities.
TEK gene mutation detection: Blood samples were collected after informed consent was obtained from the patient's guardian. The genomic DNA was extracted from the samples. A pair of customized primers was used for amplification of TEK, followed by NGS sequencing and analysis.
2.2. Laboratory tests
The laboratory findings demonstrated severe anemia (1.63 × 1012/L red blood cell and 28 g/L hemoglobin, Fig. 2) characterized by small cells and low pigment (mean corpuscular volume 17.2 pg, mean corpuscular hemoglobin concentration 252 g/L, and serum iron 1.4 μmol/L). The white blood cell count was 3.0 × 109/L and the platelet count was 119 × 109/L. The fecal occult blood test was positive. Liver function, renal function, and myocardial enzyme were nearly normal. Ultrasound revealed that the bilateral internal jugular vein was normal. The muscle tissue and subcutaneous facial and neck tissue confirmed multiple heterogeneous key vocal ranges, attributable to potential hemangioma. The lower limb venous ultrasound examination did not see any obvious anomalies. A multiple heterogeneous low echo area was found in the muscular rear of the left leg, with respect to hemangioma. In addition, the abdominal echography revealed multiple heterogeneous low echoes in liver, pancreas, retroperitoneum, also indicating hemangioma. X-ray examination found changes on the left side of the tibiofibula and soft tissue, suggesting hemangioma involving the tibiofibula.
Figure 2.
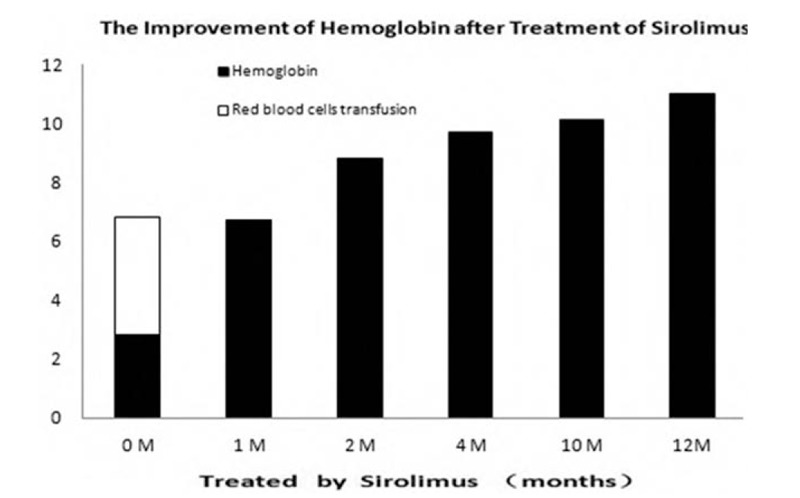
Alleviation of hemoglobin after treatment with sirolimus. White column: red blood cells transfusion (unit), black column: hemoglobin (g/mL).
Mutation analysis: Blood sample sequencing detected a heterozygous de novo mutation c.2545C > T in the TEK gene, resulting in the amino acid substitution Arg849Trp.
2.3. Sirolimus treatment
This research was approved by the Ethics Committee of Chinese PLA General Hospital, and the patient's guardian provided informed consent. At the age of 12 years, sirolimus was orally administered at an initial dosage of 1 mg/(m2 × d), average 0.7 mg/d. The blood concentrations of sirolimus remained 8.48 μg/L (6.2–11.89 μg/L). Her guardian was asked to record symptoms and side effects in detail, and blood counts and liver and renal function were regularly monitored every 3 months. The psychomotor development of the proband was also clinically assessed. The patient's hemoglobin was markedly increased from 28 g/L before treatment to 67 g/L after treatment for 1 month and 110 g/L after treatment for 1 year (Fig. 2). Since then, she did not receive any blood transfusions. The skin and mucosal hemangioma decreased significantly (Fig. 1), and no new digestive tract hemorrhage, muscle hematoma, or adverse drug reactions were observed.
Figure 1.
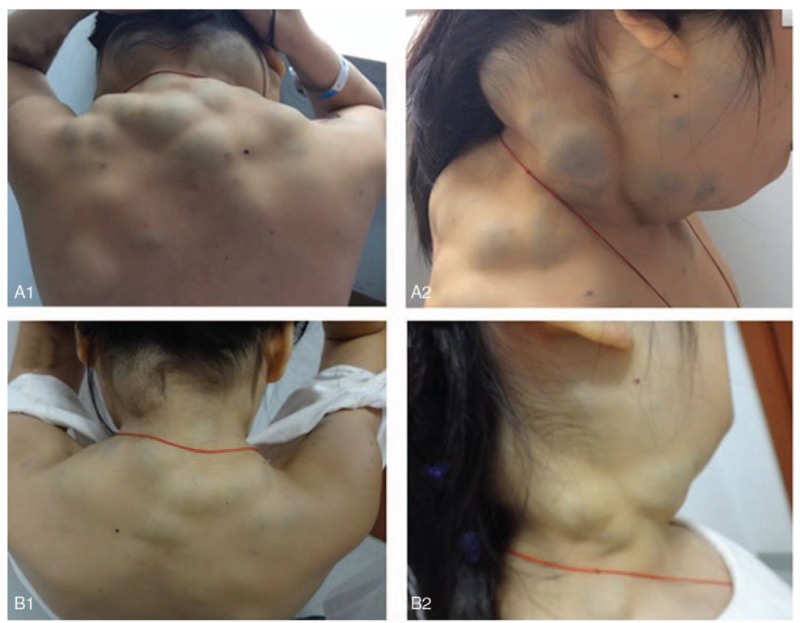
Blue rubber bleb on the back and side of the neck before and after sirolimus treatment. Blue rubber bleb on the back of the neck before treatment (A1) and after treatment (B1). Blue rubber bleb on the side of the neck before treatment (A2) and after treatment (B2).
3. Discussion
BRBNS is one of the vascular development dysplasia diseases characterized by multiple distinctive cutaneous, gastrointestinal, and many parts of the body's venous malformations. Owing to the blue nevus-like appearance, the lesion has rubbery elasticity, protrudes from the skin, which was described in detail by Bean. Therefore, the term “blue rubber bleb nevus syndrome” was coined. Venous malformations are most prominent in the skin and gastrointestinal tract; however, they have also been reported in orbital, internal organs, and bone. Thus, the involvement of these organs renders that BRBNS is associated with the development of lymphangioma.[2,9]
The pathological changes associated are primarily vascular malformation and formation of the small briquette. Furthermore, the lesions of the skin were divided into 3 types, type I lesions are large and disfiguring venous malformations; type II lesions, the most common BRBNS lesions, have a cyanotic appearance, are soft and elevated; type III lesions are irregular blue–black macules or papules.[10]
The gastrointestinal hemorrhage was an extremely common and persistent clinical symptom because of sudden massive hemorrhage, whereas venous malformations of the gastrointestinal occur rarely.[11] The most common complication is severe iron deficiency anemia, due to chronic blood loss from gastrointestinal tract; patients often need a prolonged iron treatment and sometimes regular blood transfusions to sustain life.
Herein, we report a case characterized by the appearance of multiple hemangiomas on skin upon birth; the number and volume increased with age, accompanied by digestive tract hemorrhage and severe anemia. Every 1 to 3 weeks, 2 units of red blood cells were transfused. When the female was admitted to our hospital, the hemoglobin level was 28 g/L, and the clinical manifestations were in agreement with that of BRBNS.
We speculated that the pathogenesis of BRBNS was linked to the mutations in the angiopoietin receptor TIE2/TEK on chromosomal location 9p21, leading to cutaneomucosal and venous malformations in several regions of the body. A histopathological examination revealed blood-filled vessels, lined by a single layer of endothelial cells with surrounding thin connective tissue.[12]
Wouters et al reported 12 new families with TEK mutations, and 14 patients from 6 of the families harbored R849W substitutions. The exon missense mutations were primarily in TEK exon 15 or 17 and 22. Thus, the c.2545C > T substitution was probably a hotspot region for mutations.[13]
Here, we report a case, which is also TEK exon 15 mutation, and the location is c.2545C > T mutation. Although this case is reported to exhibit an autosomal dominant transmission, most cases are sporadic. As the child's family history is not known, we are unable to determine if the mutations are hereditary from the parents’ genome, or are spontaneous genetic mutations.
However, the clinical phenotype of the case presented here was not similar to those reported by Wouters et al. Around 14 cases reported by Wouters et al were from 6 families and were p.R849W mutations, but only 2 cases were involved internal organs, lung, and bone.[13]
In addition to the location on the skin and mucous membrane, multiple hemangiomas in our case exhibited lesions widely across the organs such as liver, pancreas, left calf fibula, and back left crus muscle. Multiple organs damage is the phenotypic characteristic of our reported case. The mechanism, with respect to the same gene locus mutation but inconsistent clinical phenotype, is not clear.
In addition to realizing that the abnormal blood vessels were extensively distributed in the body, an overall check-up including that with ultrasound and magnetic resonance imaging (MRI) is crucial, in order to avoid missing-out some lesions. MRI is considered helpful in finding the visceral lesions, such as in the muscle and bone.[14,15]
Presently, there are no effective radical cure methods for BRBNS; however, a few approaches have been used, such as blood transfusion, and the complementing iron agent is utilized for the remedy of anemia. Antiangiogenic drugs such as corticosteroids, interferon, vincristine, or octreotide, are often used to treat BRBNS. These drugs can reduce the growth and proliferation of vascular cells leading to stabilization of the disease and partial remission of bleeding episodes.[12] However, these drugs are currently not recommended because of only limited success.[1] Based on the location, number, and size of vascular malformations, different surgical treatment methods may be chosen: for example, hardener therapy, surgical removal, laser, and anal colon anastomosis.[16]
Sirolimus is a novel drug to treat BRBNS. Initially, sirolimus, as an angiogenesis inhibitor, was allowed for kidney transplantation by FDA in the United States. The recommended valley concentration was 15 to 20 ng/mL; hyperlipidemia, mucositis, and poor wound healing were the main toxicities.[17]
At present, BRBNS has been reported to be successfully treated with sirolimus. We then received approval from the patient's guardian to begin low-dose sirolimus, an antiangiogenic agent. The starting dose was 1.4 mL (=1.4 mg), equivalent to 0.04 mg/kg d. After 1 month of treatment with sirolimus, the hemoglobin level started to rise, and after 1 year of treatment, the level of hemoglobin increased from 28 g/L to 110 g/L, the digestive tract did not show any repetitive bleeding, no blood transfusion was needed, and the size and number of hemangiomas decreased but not disappeared (Fig. 2). In addition, adverse drug reactions or complications were monitored, including those of hepatic, kidney, oral stomatitis, neutropenia, cholesterol levels, and systemic infection, and none were observed.
Currently, the BRBNS cases with sirolimus treatment around the world are rare, and the dose of sirolimus and the period of treatment are uncertain. However, Salloum et al[18] reported 4 cases of successful treatment with dose of siroliums range from 10 to 13 ng/mL of BRBNS Children patients aged 2 to 16 years after 18 to 26 months follow-up. Margolin et al suggested that the recommended dose of siroliums in treatment of BRBNS was 1 mg/kg/d, with daily target of 10-15 ng/mL.[19] Yuksekkaya et al[11] reported a successful treatment of an 8-year-old BRBNS girl with 1 to 5 ng/mL target dose of sirolimus after 23 months follow-up. Carlos et al[20] reported a successful treatment of a 25 year-old BRBNS female with 1 mg sirolimus twice per day after 1 year of follow-up.[20] In our case, the sirolimus blood concentration was as low as 6.2 ng/mL. The clinical effect was remarkable without side effects. However, some groups postulate that the target valley concentration for the treatment of BRBNS is 10–15ng/mL. Therefore, in order to reduce the toxic effect of sirolimus with appropriate doses, additional studies are required for increasing the experience of sirolimus treatment of BRBNS.
In conclusion, we report a case of a mutation in exon 15 of the TEK gene leading to BRBNS. It was successfully treated with a small dose of sirolimus as an alternative to blood transfusion in order to save the of BRBNS patient's life.
Footnotes
Abbreviations: BRBNS = blue rubber bleb nevus syndrome, EEG = electroencephalogram, MRI = magnetic resonance imaging.
K-LW and S-FM contributed equally to this study.
This work was supported by grants from Capital of the Public Health Program Cultivation Project (no. Z141100002114001), Major State Basic Research Development Program (973; no. 2012CB517903), and the National Natural Science Foundation of China (no. 81471329, 81211140048, 81201013, 81200463).
The authors have no conflicts of interest to disclose.
References
- [1].Ballieux F, Boon LM, Vikkula M. Blue bleb rubber nevus syndrome. Handb Clin Neurol 2015;132:223–30. [DOI] [PubMed] [Google Scholar]
- [2].Jin XL, Wang ZH, Xiao XB, et al. Blue rubber bleb nevus syndrome: a case report and literature review. World J Gastroenterol 2014;20:17254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shams PN, Cugati S, Wells T, et al. Orbital varix thrombosis and review of orbital vascular anomalies in blue rubber bleb nevus syndrome. Ophthal Plast Reconstr Surg 2014;31:e82–6. [DOI] [PubMed] [Google Scholar]
- [4].Eiríspuñal J, Picóncotos M, Visolorenzo A, et al. Epileptic disorder as the first neurologic manifestation of blue rubber bleb nevus syndrome. J Child Neurol 2002;17:219–22. [DOI] [PubMed] [Google Scholar]
- [5].Dòmini M, Aquino A, Fakhro A, et al. Blue rubber bleb nevus syndrome and gastrointestinal haemorrhage: which treatment? Eur J Pediatr Surg 2002;12:129–33. [DOI] [PubMed] [Google Scholar]
- [6].Nobuhara Y, Onoda NK, Hosomi N, et al. TIE2 gain-of-function mutation in a patient with pancreatic lymphangioma associated with blue rubber-bleb nevus syndrome: report of a case. Surg Today 2006;36:283–6. [DOI] [PubMed] [Google Scholar]
- [7].Laplante M, Sabatini D. mTOR signaling in growth control and disease. Cell 2012;149:274–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jiang BH, Liu LZ. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv Cancer Res 2009;102:19–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Marín-Manzano E, Utrilla López A, Puras Magallay E, et al. Cervical cystic lymphangioma in a patient with blue rubber bleb nevus syndrome: clinical case report and review of the literature. Ann Vasc Surg 2010;24:1–5. [DOI] [PubMed] [Google Scholar]
- [10].Gupta N, Day AS. Editorial comment: blue rubber bleb naevus syndrome. J Paediatr Child Health 2010;46:67–8. [DOI] [PubMed] [Google Scholar]
- [11].Yuksekkaya H, Ozbek O, Keser M, et al. Blue rubber bleb nevus syndrome: successful treatment with sirolimus. Pediatrics 2012;129:e1080–4. [DOI] [PubMed] [Google Scholar]
- [12].Agnese M, Cipolletta L, Bianco MA, et al. Blue rubber bleb nevus syndrome. Dermatologica 1983;167:632–5. [DOI] [PubMed] [Google Scholar]
- [13].Wouters V, Limaye N, Uebelhoer M, et al. Hereditary cutaneomucosal venous malformations are caused by TIE2 mutations with widely variable hyper-phosphorylating effects. Eur J Hum Genet 2010;18:414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Elsayes KM, Menias CO, Dillman JR, et al. Vascular malformation and hemangiomatosis syndromes: spectrum of imaging manifestations. Am J Roentgenol 2008;190:1291–9. [DOI] [PubMed] [Google Scholar]
- [15].Ganesh R, Reddy M, Janakiraman L, et al. Blue rubber bleb nevus syndrome. Indian J Pediatr 2014;81:317–8. [DOI] [PubMed] [Google Scholar]
- [16].Dasgupta R, Fishman SJ. Management of visceral vascular anomalies. Semin Pediatr Surg 2014;23:216–20. [DOI] [PubMed] [Google Scholar]
- [17].Hammill AM, Wentzel MS, Gupta A, et al. Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr Blood Cancer 2011;57:1018–24. [DOI] [PubMed] [Google Scholar]
- [18].Salloum R, Fox CE, Alvarez-Allende CR, et al. Response of blue rubber bleb nevus syndrome to sirolimus treatment. Pediatr Blood Cancer 2016;63:1911–4. [DOI] [PubMed] [Google Scholar]
- [19].Margolin JF, Soni HM, Pimpalwar S, et al. Medical therapy for pediatric vascular anomalies. Semin Plast Surg 2014;28:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Menegozzo CAM, Novo FDCF, Mori ND, et al. Postoperative disseminated intravascular coagulation in a pregnant patient with Blue Rubber Bleb Nevus Syndrome presenting with acute intestinal obstruction: case report and literature review. Int J Surg Case Rep 2017;39:235–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


