Abstract
Background:
Platelet to lymphocyte ratio (PLR) was recently reported being associated with the prognosis of pancreatic cancer (PC), but the prognostic value of PLR in pancreatic cancer remains inconsistent. We conduct a meta-analysis to evaluate the prognostic role of PLR in patients with PC.
Methods:
PubMed, Embase, Cochrane Library, and Web of Science were systematically searched for eligible studies which investigated the relationship between PLR and clinical outcome of patients with pancreatic cancer. Pooled hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated to evaluate the prognostic role of PLR in overall survival (OS) and progression-free survival (PFS)/time to progression (TTP).
Results:
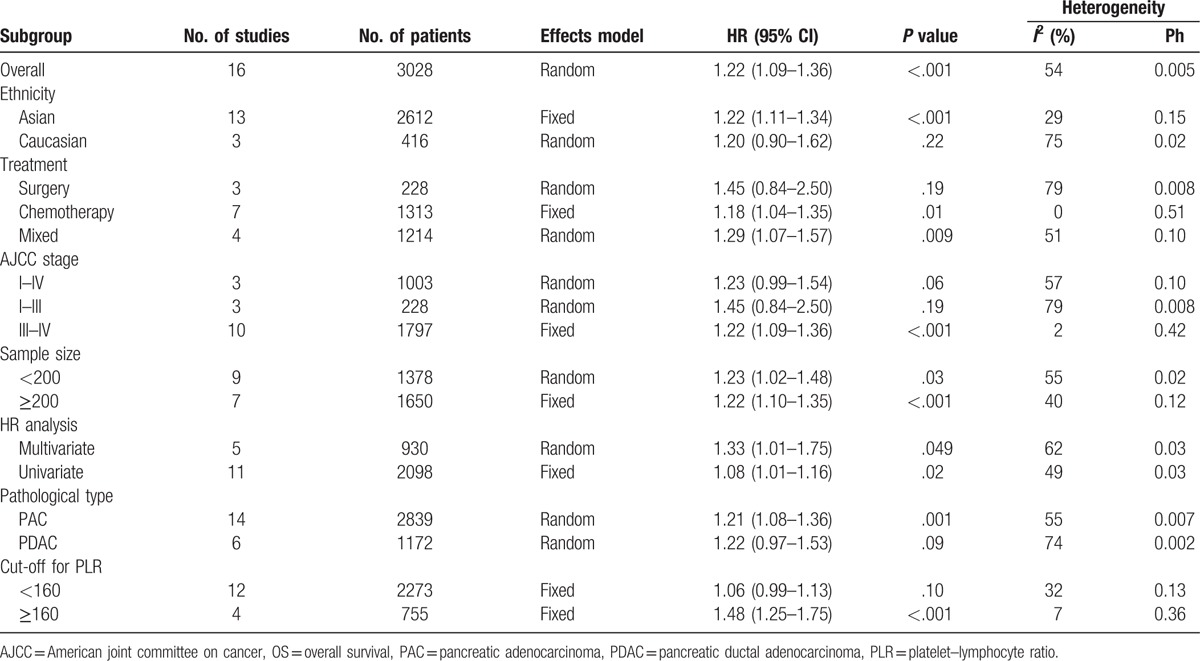
A total of 16 studies comprising 3028 patients with PC were enrolled in this meta-analysis. Pooled analysis demonstrated that elevated PLR predicted a poor OS (HR = 1.22, 95% CI: 1.09–1.36, P < .001). Prognostic role of PLR on OS were significant in subgroup of Asians (HR = 1.22, 95% CI: 1.11–1.34, P < .001), patients treated with chemotherapy (HR = 1.18, 95% CI: 1.04–1.35, P = .01) and mixed methods (HR = 1.29, 95% CI: 1.07–1.57, P = .009), American joint committee on cancer (AJCC) stage of III–IV (HR = 1.22, 95% CI: 1.09–1.36, P < .001), pathological subtype of pancreatic adenocarcinoma (HR = 1.21, 95% CI: 1.08–1.36, P = .001), and cut-off value of PLR ≥160 (HR = 1.48, 95% CI: 1.25–1.75, P < .001).
Conclusions:
An elevated PLR is associated with unfavorable overall survival in patients with pancreatic cancer.
Keywords: meta-analysis, pancreatic cancer, platelet to lymphocyte ratio, prognosis
1. Introduction
Pancreatic cancer (PC) remains an extremely aggressive malignancy, as it is the fourth leading cancer-related death worldwide.[1] Despite progress has been made in the diagnosis and therapies of PC, the prognosis of which is still poor. Besides tumor-node-metastasis (TNM) staging system, effective and convenient prognostic biomarkers are needed. Therefore, it is of paramount importance for us to identify a better prognostic biomarker for prognosis of PC, which could facilitate the selection of individual therapeutic strategy in clinical management.
Recently, systemic inflammation status has been revealed to be involved in tumor development and progression.[2–4] Serum biomarkers of inflammation can be readily obtained through peripheral blood samples, such as C-reactive protein, neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), and platelet to lymphocyte ratio (PLR). Such biomarkers have been revealed to have prognostic value for a variety of malignancies, including colorectal cancer,[5] lung cancer,[6] ovarian cancer,[7] and so on. A multitude of studies indicated that an elevated NLR predicted poor survival in patients with PC.[8–12] Evidence has suggested that LMR was associated with favorable survival in PC patients.[13–16] Nevertheless, the prognostic value of PLR for PC patients is still controversial and has not yet been systematic analyzed. Therefore, we conducted this meta-analysis to elucidate the prognostic value of PLR on OS and progression-free survival (PFS)/time to progression (TTP) in PC patients. As far as we know, this is the first meta-analysis explored the connection between PLR and prognosis in PC.
2. Materials and methods
2.1. Search strategies
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[17] We performed a comprehensive literature search of PubMed, Embase, Cochrane Library, and Web of Science up to June 30, 2017. The following terms were used: “PLR” (e.g., “platelet lymphocyte ratio,” “platelet–lymphocyte ratio,” “platelet to lymphocyte ratio,” and “platelet-to-lymphocyte ratio”) and “PC” (e.g., “pancreatic cancer,” “pancreatic carcinoma,” “pancreatic tumor,” “pancreatic neoplasms,” “pancreatic adenocarcinoma,” and “PDAC,”). References of relevant studies were also checked for eligible studies. This study was approved by the Clinical Ethics Committee of Peking University Third Hospital.
2.2. Inclusion and exclusion criteria
Inclusion criteria for selecting the studies were as follows: patients with PC were pathological examination confirmed; studies described the correlation of PLR with overall survival (OS) and/or cancer-specific survival (CSS) and/or time to progression (TTP) and/or disease-free survival (DFS) and/or progression-free survival (PFS); hazard ratios (HRs) with 95% confidence intervals (95% CIs) were reported. Exclusion criteria were: letters, case reports, abstracts, editorials, comments, and reviews; studies with duplicate data and repeat analyses; studies were not written in English.
2.3. Data extraction
The following information was captured by 2 independent authors (WL and LT): first author's name, year of publication, country, ethnicity, survival analysis methods (multivariate/univariate), time of follow-up and survival outcome (OS, CSS, PFS, and TTP). Age of patients, sample size (male and female), pathological subtype, American joint committee on cancer (AJCC) stage, treatment strategy, cut-off values of PLR, and consideration of receiver operating characteristic (ROC) curves for selection of cut-off. HRs as well as their 95%CIs for OS, CSS, PFS, and TTP. HRs and their 95%CIs were extracted from multivariable analyses in consideration of confounding factors. If multivariable analyses were not available, HRs from univariable analyses were extracted. Any discrepancies were resolved by discussion to reach a consensus.
2.4. Qualitative assessment
The quality assessment of each study was independently performed by 2 authors (WL and ML) according to the Newcastle-Ottawa Scale (NOS),[18] which included criteria of sample selection (0–4 points), comparability (0–2 points), and outcome assessment (0–3 points). Studies with NOS score of ≥6 were assigned as high-quality studies.
2.5. Statistical analysis
STATA version 13.0 (StataCorp, CollegeStation, TX) was used for statistical analysis. The chi-square-based Q-statistic test and the I-squared statistic were performed to assess the inter-study heterogeneity.[19] A fixed-effects model was used if there was no significant heterogeneity (Ph >0.10 for the Q-test and I2 <50%). Otherwise, a random-effects model was selected. The aggregated HRs and 95% CIs were applied to access the prognostic value of PLR on OS and PFS/TTP. Subgroup analyses were conducted on the following items to explore the heterogeneity among the results of different studies: ethnicity, treatment, AJCC stage, sample size, HR analysis method, pathological type, and cut-off for PLR. Sensitivity analyses were conducted by evaluating result stability after sequential omission of each study. Egger linear regression test was used to estimate publication bias.[20] A trim and fill method was applied to estimate asymmetry in the funnel plot.[21] All statistical tests were 2-tailed, and P < .05 was defined as statistical significance.
3. Results
3.1. Search results and study characteristics
The initial search algorithm retrieved a total of 68 studies, of which 7 were duplicates. After titles and abstracts assessed for eligibility, the left 32 articles were enrolled for full-text articles screened. Of them, 15 articles were excluded due to conference abstract or insufficient data. Finally, we selected 17 studies[9,22–37] for the meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[17] The processes of study selection were shown in the PRISMA flow diagram (Fig. 1).
Figure 1.
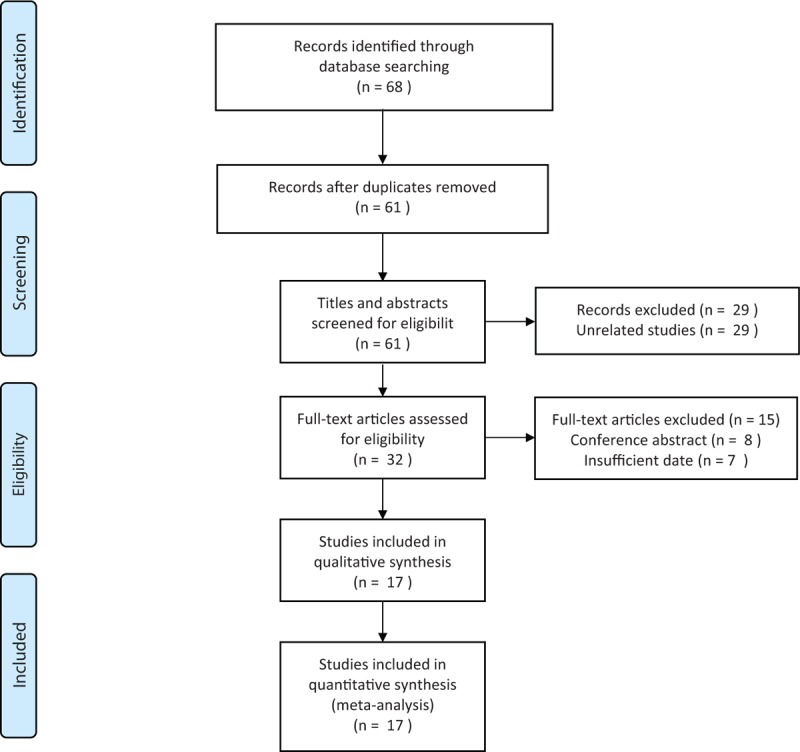
Flow diagram of the study selection in the analysis.
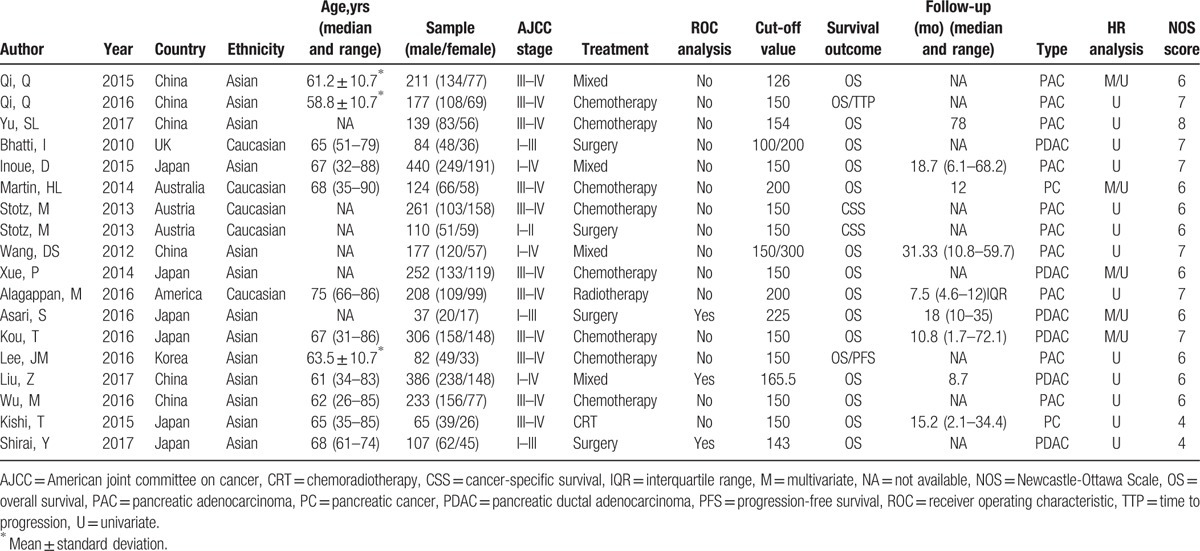
Seventeen studies comprising 3028 patients were included for the present analysis and characteristics are summarized in Table 1. Qi et al[22,23] reported 2 independent studies, and samples came from the same institution in China, but the enrolled patients were non-repetitive. Stotz et al[37] reported 2 groups of pancreatic cancer patients, in which the relationship between the PLR and CSS was available. These studies were published between 2010 and 2017 and contained sample sizes ranged from 37 to 440. There were 13 studies evaluated Asian patients, and the other 4 evaluated Caucasian patients. “Caucasian” means Caucasian race and the majority of the patients enrolled in non-Asian studies were Caucasian. The treatments were surgery, chemotherapy, radiotherapy, chemoradiotherapy, and mixed methods. There were 16 studies reported the association between PLR and OS, and 2 studies investigated prognostic value of PLR for PFS/TTP. Cutoff values of PLR ranged from 126 to 300. The Newcastle-Ottawa scale (NOS) scores of the studies varied from 6 to 8.[18]
Table 1.
Main characteristics of all the studies included in the meta-analysis.
3.2. Meta-analysis
3.2.1. The prognostic value of PLR in OS
Sixteen studies comprising 3028 patients reported the association between PLR and OS. The pooled analysis suggested that an elevated PLR predicted an unfavorable OS (HR = 1.22, 95% CI: 1.09–1.36, P < .001). There was heterogeneity of included studies and a random-effects model was used (Ph = 0.005; I2 = 54%) (Fig. 2).
Figure 2.
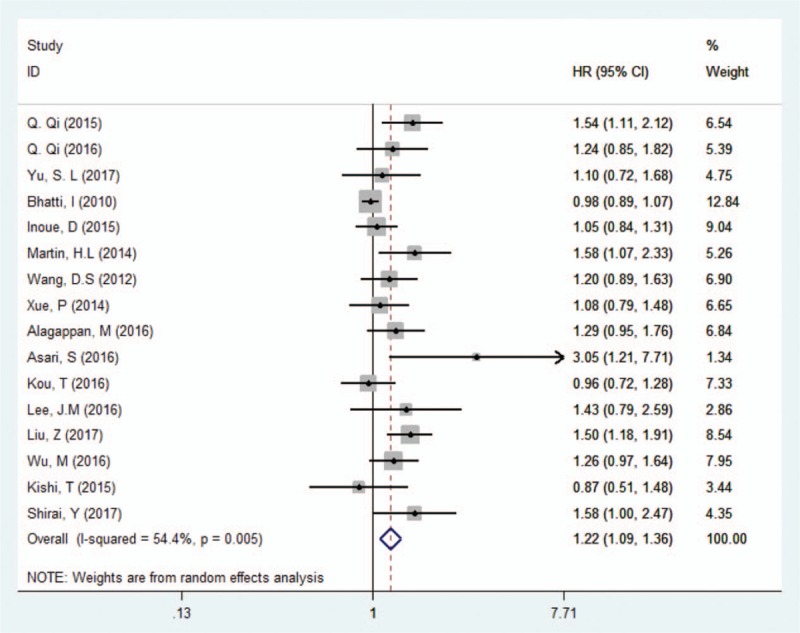
Forest plots for the association between PLR and OS in PC. OS = overall survival, PC = pancreatic cancer, PLR = platelet–lymphocyte ratio.
3.2.2. The prognostic value of PLR in PFS/TTP
Two studies comprising 259 patients evaluated the relationship between PLR and PFS/TTP. The fixed-effects model was used for analysis because there was no significant heterogeneity (Ph = 0.698; I2 = 0%). Pooled data indicated that PLR has no prognostic value for PFS/TTP in patients with advanced pancreatic adenocarcinoma (HR = 1.29, 95% CI: 0.94–1.79, P = .24) (Fig. 3).
Figure 3.
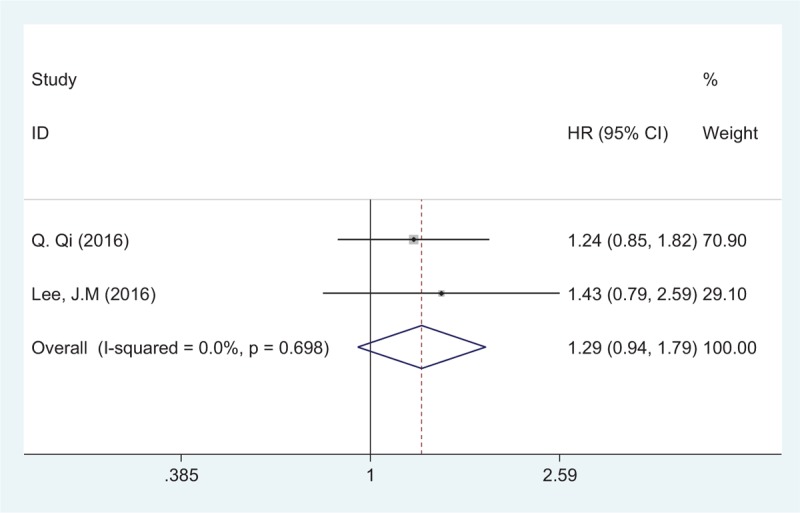
Forest plots for the association between PLR and PFS/TTP. PFS/TTP = progression-free survival/time to progression, PLR = platelet–lymphocyte ratio.
3.2.3. Subgroup analysis
We conducted subgroup analyses for PLR and OS based on ethnicity, treatment, AJCC stage, sample size, HR analysis method, pathological type, and cut-off for PLR (Table 2). Subgroup analysis according to ethnicity showed that PLR had a prognostic role for OS in Asians (HR = 1.22, 95% CI: 1.11–1.34, P < .001). In the subgroup of the treatment, increased PLR predicted a pool OS in patients treated with chemotherapy (HR = 1.18, 95% CI: 1.04–1.35, P = .01) and mixed methods (HR = 1.29, 95% CI: 1.07–1.57, P = .009). When stratified by AJCC stage, the analyses indicated that PLR was a prognostic marker in patients with AJCC stage of III–IV (HR = 1.22, 95% CI: 1.09–1.36, P < .001). Subgroup analysis for pathological type indicated that PLR had prognostic value for pancreatic adenocarcinoma (HR = 1.21, 95% CI: 1.08–1.36, P = .001). In the subgroup of the cut-off value for PLR, the results indicated that increased PLR was significantly associated with unfavorable OS in studies of cut-off value ≥160 (HR = 1.48, 95% CI: 1.25–1.75, P < .001). PLR showed prognostic value regardless of sample size and HR analysis method. Details are shown in Table 2.
Table 2.
Subgroup analyses for the association between PLR and OS.
3.3. Sensitivity analysis
Sensitivity analysis was performed to assess the stability of the results. Each single study was removed to check the influence of individual data sets on the pooled HR of OS. The result showed that the combined HR was not obviously influenced by any single study, which indicated the robustness of the outcome of the study (Fig. 4).
Figure 4.
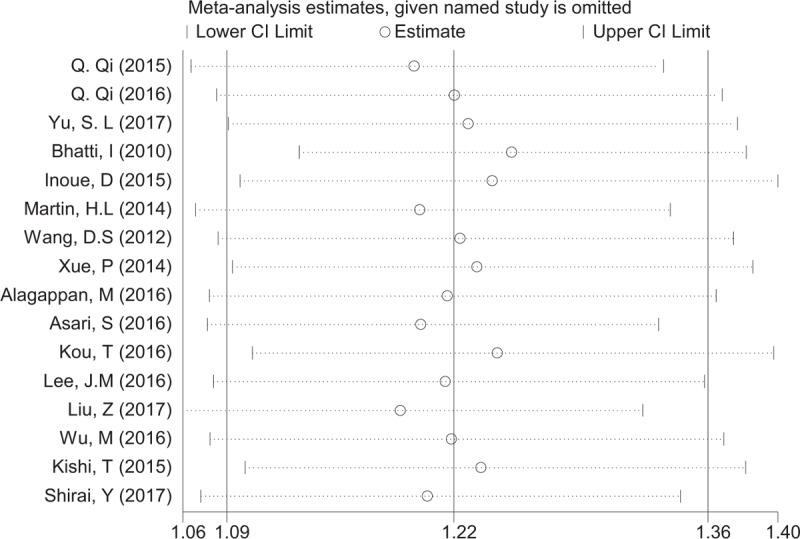
Sensitivity analyses for confirming robustness of OS by removing 1 study each time. OS = overall survival.
3.4. Publication bias
Egger publication bias plot test was used to estimate the publication bias (Fig. 5). There was publication bias for studies investigated OS in PC (P = .003). Therefore, a trim and fill method was used to fill unpublished studies, and the recalculated combined HR of OS did not be significantly affected by filling 3 unpublished studies (HR = 1.16; 95% CI, 1.04–1.30; P = .006; Fig. 6).
Figure 5.
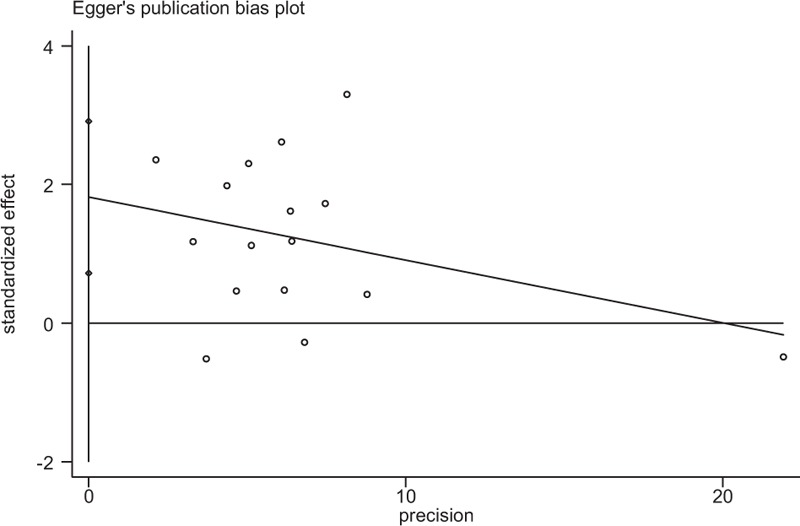
Egger publication bias plot for studies investigated OS in PC. (P = .003). OS = overall survival; PC = pancreatic cancer.
Figure 6.
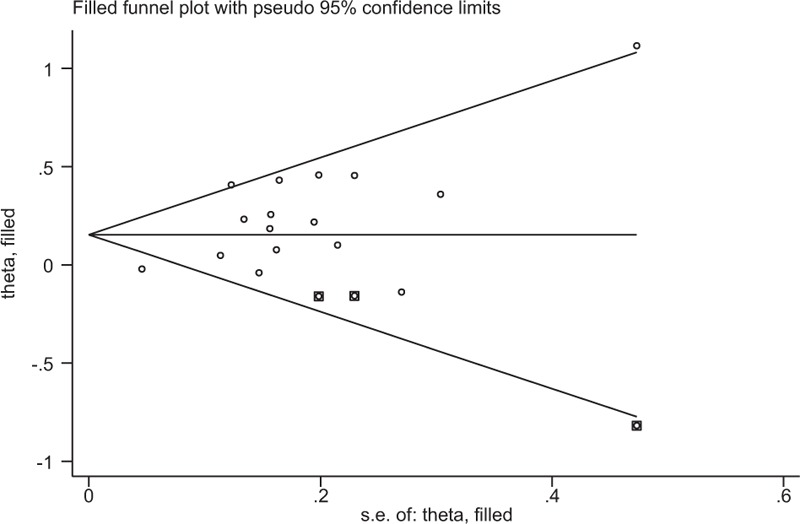
Funnel plot adjusted using a trim and fill method for studies investigated OS in PC. Diamonds: included studies; diamonds in squares: presumed missing studies. OS = overall survival; PC = pancreatic cancer.
4. Discussion
PLR was being confirmed as a significant prognostic marker in colorectal cancer (CRC) and non-small cell lung cancer(s) (NSCLC). Tan et al[38] conducted a systematic review and meta-analysis to prove that peripheral blood PLR can be used as a predictor of OS in patients with CRC. Zhao et al[39] also performed a meta-analysis suggested that elevated PLR might be a predicative factor of poor prognosis for NSCLC patients.
Our meta-analysis combined 16 studies involving 3028 patients and indicated that the elevated PLR significantly predicted unfavorable OS in patients with PC. Subgroup analyses suggested that increased PLR was associated with poor OS in Asian populations. Furthermore, subgroup analyses for treatments implied that higher prognostic value of PLR in OS was found in patients treated with chemotherapy and mixed methods. In addition, the stratified analyses showed that PLR have higher prognostic value for OS in PC patients with AJCC stage of III–IV. As for pathological subtype of PC, higher negative effect of elevated PLR on OS was observed in patients with pancreatic adenocarcinoma (PAC), and the prognostic value of PLR was impaired for pancreatic ductal adenocarcinoma (PDAC). The cut-off value of PLR for OS ranged from 126 to 300, and our stratified analyses suggested that PLR with cut-off value ≥160 may have more discriminative prognostic value for OS. Owing to the limited studies, the meta-analysis did not demonstrate prognostic value of PLR with PFS/TTP in PC.
The bilateral influence of systemic inflammatory response and tumor progression is involved in the prognosis of PC patients.[40] The exact mechanisms by which PLR predicts OS of PC patients are still undefined. Platelets are recruited and adhere to tumor cells through platelet receptors, such as glycoprotein IIb/IIIa and P-selectin.[41,42] Additionally, platelets secrete tumor growth factors, such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), transforming growth factor-β1 (TGFβ1), and insulin-like growth factor-1 (IGF1).[43] These factors play a crucial role in tumorigenesis, angiogenesis, and metastasis. Furthermore, platelets help tumor cells escape from the immune system by surrounding circulating tumor cells (CTCs) through platelets aggregation.[44]
Lymphocytes are essential components of the immune system. Tumor-infiltrating lymphocytes (TILs) are vital components of the antitumor immune microenvironment, and are cellular basis of immunosurveillance against tumor cells.[45] CD4+ and CD8+ T lymphocytes induce cytotoxic cell death and inhibit tumor cell proliferation and migration in antitumor immunitive reaction.[46,47] Furthermore, lymphocytopenia has been clarified to be a prognostic factor of OS in PC.[48] Therefore, the ratio of increased platelets and decreased lymphocytes has prognostic value for patients with PC. According to the present research, the association of platelet and lymphocyte in pancreatic cancers is that platelet help tumor cells escape from the immune system which based on lymphocyte by surrounding CTCs through platelets aggregation.
There were several limitations to this study. First, all enrolled studies were retrospective, which may exist some biases. Second, heterogeneity was found among studies for OS. Therefore, subgroup analyses were conducted to adjust for heterogeneity. Most of the studies used univariate analysis, so there was not enough evidence to prove that PLR was an independent prognostic factor for PC. Third, potential publication bias was observed in OS meta-analysis. However, through trim and fill analysis, the recalculated results confirmed the robustness of the prognostic role of PLR in OS. Finally, only English publications were enrolled, language bias cannot be excluded.
In conclusion, an elevated PLR is associated with unfavorable OS in patients with pancreatic cancer. The PLR could be a convenient and economical prognostic biomarker of pancreatic cancer in the clinical practice, which could facilitate the selection of individual therapeutic strategy for patients with PC.
Footnotes
Abbreviations: AJCC = American joint committee on cancer, CI = confidence interval, HR = hazard ratio, OS = overall survival, PAC = pancreatic adenocarcinoma, PC = pancreatic cancer, PDAC = pancreatic ductal adenocarcinoma, PFS = progression-free survival, PLR = platelet–lymphocyte ratio, TTP = time to progression.
This study was supported by a grant from the National Natural Science Foundation of China (No 81672862), and the Capital Characteristic Clinical Application Research and Achievement Promotion Project (No Z171100001017121).
The authors have no conflicts of interest to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- [3].Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shibutani M, Maeda K, Nagahara H, et al. The prognostic significance of a postoperative systemic inflammatory response in patients with colorectal cancer. World J Surg Oncol 2015;13:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yuan C, Li N, Mao X, et al. Elevated pretreatment neutrophil/white blood cell ratio and monocyte/lymphocyte ratio predict poor survival in patients with curatively resected non-small cell lung cancer: results from a large cohort. Thorac Cancer 2017;8:350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Asher V, Lee J, Innamaa A, et al. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol 2011;13:499–503. [DOI] [PubMed] [Google Scholar]
- [8].Asaoka T, Miyamoto A, Maeda S, et al. Prognostic impact of preoperative NLR and CA19-9 in pancreatic cancer. Pancreatology 2016;16:434–40. [DOI] [PubMed] [Google Scholar]
- [9].Alagappan M, Pollom EL, von Eyben R, et al. Albumin and neutrophil-lymphocyte ratio (NLR) predict survival in patients with pancreatic adenocarcinoma treated with SBRT. Am J Clin Oncol 2016;00:1–6. [DOI] [PubMed] [Google Scholar]
- [10].An X, Ding PR, Li YH, et al. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers 2010;15:516–22. [DOI] [PubMed] [Google Scholar]
- [11].Chen Y, Yan H, Wang Y, et al. Significance of baseline and change in neutrophil-to-lymphocyte ratio in predicting prognosis: a retrospective analysis in advanced pancreatic ductal adenocarcinoma. Sci Rep 2017;7:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Formica V, Morelli C, Ferroni P, et al. Neutrophil/lymphocyte ratio helps select metastatic pancreatic cancer patients benefitting from oxaliplatin. Cancer Biomark 2016;17:335–45. [DOI] [PubMed] [Google Scholar]
- [13].Fujiwara Y, Misawa T, Shiba H, et al. Postoperative peripheral absolute blood lymphocyte-to-monocyte ratio predicts therapeutic outcome after pancreatic resection in patients with pancreatic adenocarcinoma. Anticancer Res 2014;34:5163–8. [PubMed] [Google Scholar]
- [14].Li GJ, Xu HW, Ji JJ, et al. Prognostic value of preoperative lymphocyte-to-monocyte ratio in pancreatic adenocarcinoma. Onco Targets Ther 2016;9:1085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Singh G, Nassri A, Kim D, et al. Lymphocyte-to-monocyte ratio can predict mortality in pancreatic adenocarcinoma. World J Gastrointest Pharmacol Ther 2017;8:60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stotz M, Szkandera J, Stojakovic T, et al. The lymphocyte to monocyte ratio in peripheral blood represents a novel prognostic marker in patients with pancreatic cancer. Clin Chem Lab Med 2015;53:499–506. [DOI] [PubMed] [Google Scholar]
- [17].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. [DOI] [PubMed] [Google Scholar]
- [18].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [19].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [22].Qi Q, Geng Y, Sun M, et al. Clinical implications of systemic inflammatory response markers as independent prognostic factors for advanced pancreatic cancer. Pancreatology 2015;15:145–50. [DOI] [PubMed] [Google Scholar]
- [23].Qi Q, Zhuang L, Shen Y, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer 2016;122:2158–67. [DOI] [PubMed] [Google Scholar]
- [24].Yu SL, Xu LT, Qi Q, et al. Serum lactate dehydrogenase predicts prognosis and correlates with systemic inflammatory response in patients with advanced pancreatic cancer after gemcitabine-based chemotherapy. Sci Rep 2017;7:45194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bhatti I, Peacock O, Lloyd G, et al. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg 2010;200:197–203. [DOI] [PubMed] [Google Scholar]
- [26].Inoue D, Ozaka M, Matsuyama M, et al. Prognostic value of neutrophil-lymphocyte ratio and level of C-reactive protein in a large cohort of pancreatic cancer patients: a retrospective study in a single institute in Japan. Jpn J Clin Oncol 2015;45:61–6. [DOI] [PubMed] [Google Scholar]
- [27].Martin HL, Ohara K, Kiberu A, et al. Prognostic value of systemic inflammation-based markers in advanced pancreatic cancer. Intern Med J 2014;44:676–82. [DOI] [PubMed] [Google Scholar]
- [28].Wang DS, Luo HY, Qiu MZ, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol 2012;29:3092–100. [DOI] [PubMed] [Google Scholar]
- [29].Xue P, Kanai M, Mori Y, et al. Neutrophil-to-lymphocyte ratio for predicting palliative chemotherapy outcomes in advanced pancreatic cancer patients. Cancer Med 2014;3:406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Asari S, Matsumoto I, Toyama H, et al. Preoperative independent prognostic factors in patients with borderline resectable pancreatic ductal adenocarcinoma following curative resection: the neutrophil-lymphocyte and platelet-lymphocyte ratios. Surg Today 2016;46:583–92. [DOI] [PubMed] [Google Scholar]
- [31].Kou T, Kanai M, Yamamoto M, et al. Prognostic model for survival based on readily available pretreatment factors in patients with advanced pancreatic cancer receiving palliative chemotherapy. Int J Clin Oncol 2016;21:118–25. [DOI] [PubMed] [Google Scholar]
- [32].Lee JM, Lee HS, Hyun JJ, et al. Prognostic value of inflammation-based markers in patients with pancreatic cancer administered gemcitabine and erlotinib. World J Gastrointest Oncol 2016;8:555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liu Z, Jin K, Guo M, et al. Prognostic value of the CRP/Alb ratio, a novel inflammation-based score in pancreatic cancer. Ann Surg Oncol 2017;24:561–8. [DOI] [PubMed] [Google Scholar]
- [34].Wu M, Guo J, Guo L, et al. The C-reactive protein/albumin ratio predicts overall survival of patients with advanced pancreatic cancer. Tumour Biol 2016;37:12525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kishi T, Nakamura A, Itasaka S, et al. Pretreatment C-reactive protein level predicts outcome and patterns of failure after chemoradiotherapy for locally advanced pancreatic cancer. Pancreatology 2015;15:694–700. [DOI] [PubMed] [Google Scholar]
- [36].Shirai Y, Shiba H, Haruki K, et al. Preoperative platelet-to-albumin ratio predicts prognosis of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Anticancer Res 2017;37:787–93. [DOI] [PubMed] [Google Scholar]
- [37].Stotz M, Gerger A, Eisner F, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer 2013;109:416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tan D, Fu Y, Su Q, et al. Prognostic role of platelet-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhao QT, Yuan Z, Zhang H, et al. Prognostic role of platelet to lymphocyte ratio in non-small cell lung cancers: a meta-analysis including 3,720 patients. Int J Cancer 2016;139:164–70. [DOI] [PubMed] [Google Scholar]
- [40].Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493–503. [DOI] [PubMed] [Google Scholar]
- [41].Goubran HA, Stakiw J, Radosevic M, et al. Platelets effects on tumor growth. Semin Oncol 2014;41:359–69. [DOI] [PubMed] [Google Scholar]
- [42].Chen M, Geng JG. P-selectin mediates adhesion of leukocytes, platelets, and cancer cells in inflammation, thrombosis, and cancer growth and metastasis. Arch Immunol Ther Exp (Warsz) 2006;54:75–84. [DOI] [PubMed] [Google Scholar]
- [43].Qi C, Li B, Guo S, et al. P-selectin-mediated adhesion between platelets and tumor cells promotes intestinal tumorigenesis in Apc(Min/+) mice. Int J Biol Sci 2015;11:679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Amo L, Tamayo-Orbegozo E, Maruri N, et al. Involvement of platelet-tumor cell interaction in immune evasion. Potential role of podocalyxin-like protein 1. Front Oncol 2014;4:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004;21:137–48. [DOI] [PubMed] [Google Scholar]
- [46].Zikos TA, Donnenberg AD, Landreneau RJ, et al. Lung T-cell subset composition at the time of surgical resection is a prognostic indicator in non-small cell lung cancer. Cancer Immunol Immunother 2011;60:819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Minami T, Minami T, Shimizu N, et al. Identification of programmed death ligand 1-derived peptides capable of inducing cancer-reactive cytotoxic T lymphocytes from HLA-A24+ patients with renal cell carcinoma. J Immunother 2015;38:285–91. [DOI] [PubMed] [Google Scholar]
- [48].Fogar P, Sperti C, Basso D, et al. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas 2006;32:22–8. [DOI] [PubMed] [Google Scholar]


