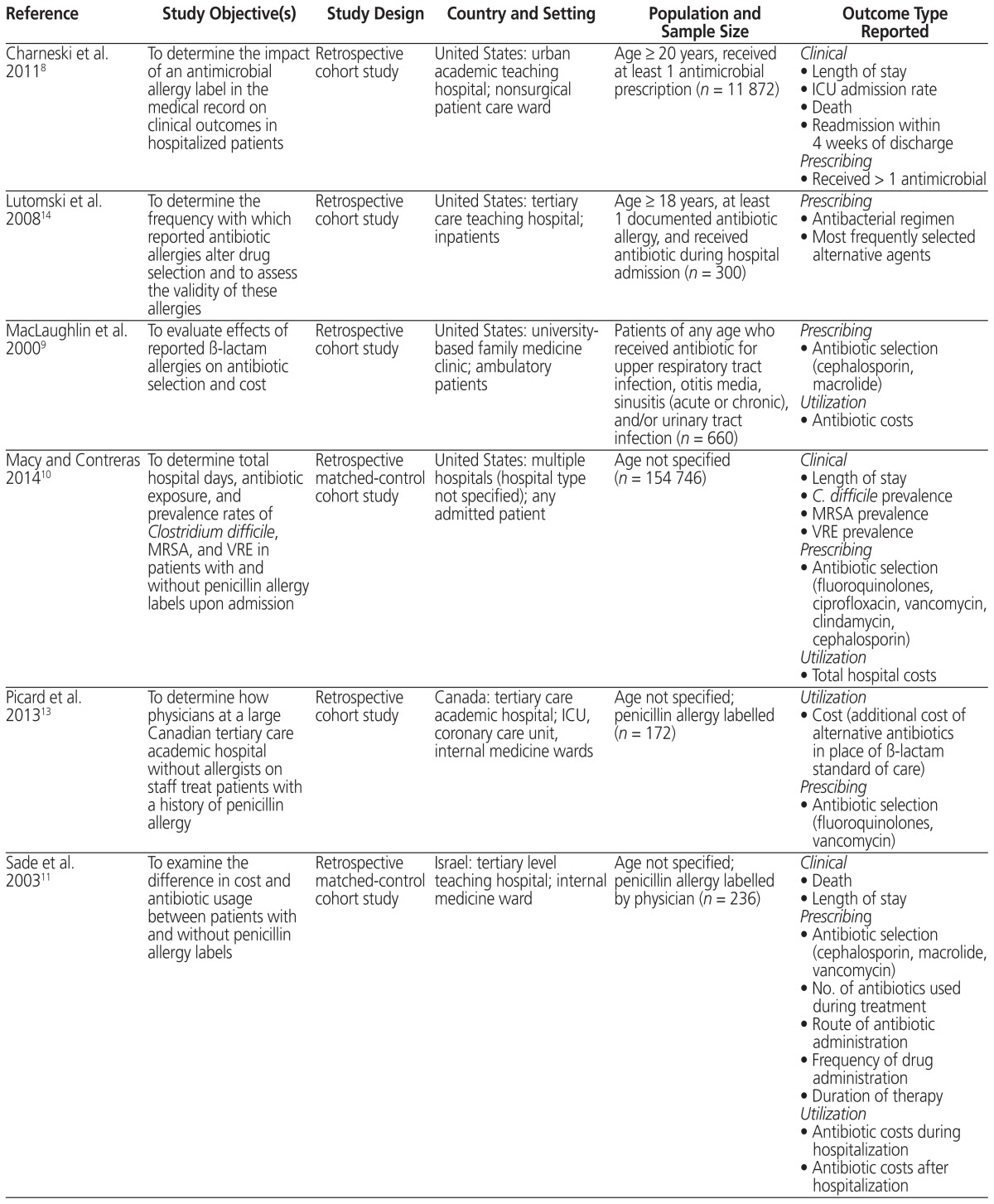
Table 1.
Summary of Studies Included in a Systematic Review of Antimicrobial Allergy Labelling
| Reference | Study Objective(s) | Study Design | Country and Setting | Population and Sample Size | Outcome Type Reported |
|---|---|---|---|---|---|
| Charneski et al. 20118 | To determine the impact of an antimicrobial allergy label in the medical record on clinical outcomes in hospitalized patients | Retrospective cohort study | United States: urban academic teaching hospital; nonsurgical patient care ward | Age ≥ 20 years, received at least 1 antimicrobial prescription (n = 11 872) |
Clinical
|
| Lutomski et al. 200814 | To determine the frequency with which reported antibiotic allergies alter drug selection and to assess the validity of these allergies | Retrospective cohort study | United States: tertiary care teaching hospital; inpatients | Age ≥ 18 years, at least 1 documented antibiotic allergy, and received antibiotic during hospital admission (n = 300) |
Prescribing
|
| MacLaughlin et al. 20009 | To evaluate effects of reported β-lactam allergies on antibiotic selection and cost | Retrospective cohort study | United States: university- based family medicine clinic; ambulatory patients | Patients of any age who received antibiotic for upper respiratory tract infection, otitis media, sinusitis (acute or chronic), and/or urinary tract infection (n = 660) |
Prescribing
|
| Macy and Contreras 201410 | To determine total hospital days, antibiotic exposure, and prevalence rates of Clostridium difficile, MRSA, and VRE in patients with and without penicillin allergy labels upon admission | Retrospective matched-control cohort study | United States: multiple hospitals (hospital type not specified); any admitted patient | Age not specified (n = 154 746) |
Clinical
|
| Picard et al. 201313 | To determine how physicians at a large Canadian tertiary care academic hospital without allergists on staff treat patients with a history of penicillin allergy | Retrospective cohort study | Canada: tertiary care academic hospital; ICU, coronary care unit, internal medicine wards | Age not specified; penicillin allergy labelled (n = 172) |
Utilization
|
| Sade et al. 200311 | To examine the difference in cost and antibiotic usage between patients with and without penicillin allergy labels | Retrospective matched-control cohort study | Israel: tertiary level teaching hospital; internal medicine ward | Age not specified; penicillin allergy labelled by physician (n = 236) |
Clinical
|
| Trubiano et al. 20155 | To examine the rate of antimicrobial allergy labelling at a tertiary referral centre and impacts on antimicrobial usage and appropriateness | Two inpatient antimicrobial prevalence surveys | Australia: tertiary referral centre; inpatients | Inclusion criteria not specified (n = 509) |
Prescribing
|
| Trubiano et al. 201512 | To (1) determine the prevalence of antimicrobial allergy labels in patients with cancer; (2) provide a description of reported antibiotic allergies; and (3) describe the impacts of an antimicrobial allergy label on antimicrobial choice, usage, and clinical outcomes | Retrospective cohort study | Australia: tertiary referral centre for cancer patients; oncology, hematology | Age not specified; patients coded as having an infective diagnosis who received antimicrobial agent for treatment of infection, with inpatient admission > 24 h (n = 198) |
Clinical
|
ICU = intensive care unit, MRSA = multidrug-resistant Staphylococcus aureus, VRE = vancomycin-resistant Enterococcus.