Figure 1. NAD+ intermediates, biosynthetic enzymes, and downstream mediators.
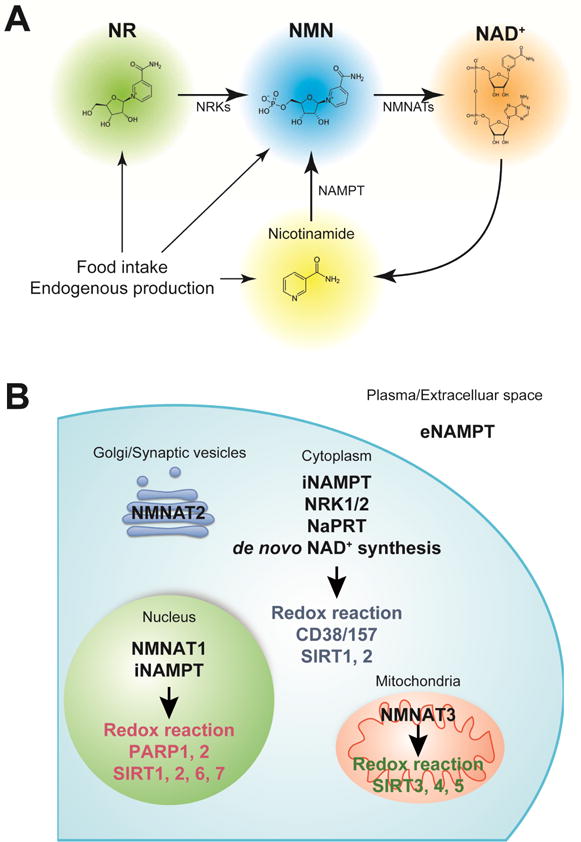
(A) Nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR) are natural compounds that effectively enhance NAD+ biosynthesis and have health benefits. In mammals, NMN is synthesized from nicotinamide by the rate-limiting enzyme, nicotinamide phosphoribosyltransferase (NAMPT). NMN is also synthesized from NR via an NR kinase (NRK)-mediated phosphorylation reaction. NMN is then converted into NAD+ by NMN adenylyltransferases (NMNATs).
(B) NAMPT has two distinct forms: intracellular and extracellular NAMPT (iNAMPT and eNAMPT, respectively). iNAMPT is present in the cytoplasm and nucleus. eNAMPT is secreted by multiple cell types, including adipocytes and immune cells. eNAMPT is fully active as an NAD+ biosynthetic enzyme capable of catalyzing the generation of NMN, whereas it has also been reported to have a role as an inflammatory cytokine that are independent of catalytic activity. Nicotinic acid phosphoribosyltransferase (NaPRT) catalyzes the first step in the Preiss-Handler pathway, which converts nicotinic acid to NAD+, and de novo synthesis from tryptophan occurs through a complex series of steps ending with conversion of nicotinic acid adenine dinucleotide (NaAD+) to NAD+ by glutamine-dependent NAD+ synthase. NRK1 and NRK2 convert NR to NMN, which can then be acted on by NMNAT1–3. NMNAT1 is exclusively nuclear. NMNAT2 has been reported to localize to the Golgi complex and synaptic vesicles, and is predominantly expressed in neurons. NMNAT3 is the only NAD+ biosynthetic enzyme shown to be localized in the mitochondrial matrix, except in erythrocytes where it is expressed in the cytosol. NAD+ is consumed by NAD+-dependent enzymes, such as poly-ADP-ribose polymerases (PARPs), sirtuins, CD38/157, and other NAD+ glycohydrolases, and redox reaction.
