Abstract
Advancements in rectal cancer treatment have resulted in improvement only in locoregional control and have failed to address distant relapse, which is the predominant mode of treatment failure in rectal cancer. As the efficacy of conventional chemoradiotherapy (CRT) followed by total mesorectal excision (TME) reaches a plateau, the need for alternative strategies in locally advanced rectal cancer (LARC) has grown in relevance. Several novel strategies have been conceptualized to address this issue, including: 1) neoadjuvant induction and consolidation chemotherapy before CRT; 2) neoadjuvant chemotherapy alone to avoid the sequelae of radiation; and 3) nonoperative management for patients who achieved pathological or clinical complete response after CRT. This article explores the issues, recent advances and paradigm shifts in the management of LARC and emphasizes the need for a personalized treatment plan for each patient based on tumor stage, location, gene expression and quality of life.
Keywords: Locally advanced rectal cancer, neoadjuvant treatment, chemoradiotherapy
Introduction
Colorectal cancer is the third most common cancer worldwide and accounts for 8% of all cancer-related deaths (1). Globally, an estimated 1.57 million patients are diagnosed, and over 771,000 are expected to die from colorectal cancer annually (2). The worldwide incidence of colorectal cancer is increasing, with the highest increases seen in Asian countries. In contrast, decreasing mortality rates have been observed worldwide, most likely due to implementation of mandatory screening and enhanced treatment (1).
Notwithstanding advances in treatment, rectal cancer that extends beyond the rectal wall or involving locoregional lymph nodes has been challenging to cure. The boundaries of the bony pelvis, the need to safeguard nerve function, and the proximity of distal tumors to the sphincters make surgical extirpation a cumbersome endeavor (3). Given the challenging nature of rectal cancer, a multidisciplinary approach has been employed to achieve optimal outcomes with the goal of reducing the risk of distant recurrence and enhancing sphincter preservation. The current standard of care for locally advanced rectal cancer (LARC) is a multimodal approach incorporating neoadjuvant long-course chemoradiotherapy (LCRT) or neoadjuvant hypofractionated short-course radiotherapy (SCRT) followed by total mesorectal excision (TME) and adjuvant fluoropyrimidine-based chemotherapy. This approach has significantly improved local control and recurrence rates; however, an overall survival (OS) benefit has not been established (4). The use of enhanced imaging modalities has substantially improved patient stratification in LARC. Magnetic resonance imaging (MRI) provides high tissue resolution of the extent of tumor involvement and delineation of the circumferential resection margin (CRM). The CRM, the tumor distance from the mesorectal fascia, has become one of the most important parameters in evaluating patients with LARC, with a CRM>1 mm signifying good prognosis (5,6). Similarly, extramural vascular invasion (EMVI), the direct tumoral invasion of blood vessels, emerged as an important MRI prognostic feature, indicating hematogenous spread and reduced survival (6).
Refinements in surgical techniques, enhanced imaging modalities, improved surgical pathology and use of neoadjuvant therapy have contributed significantly in advancing LARC treatment; however, these advances also generated multiple issues, which continue to dominate the research agenda, and caused paradigm shifts in LARC management. Moreover, the advancements in rectal cancer treatment have resulted in improvement only in locoregional control and failed to address distant relapse, which is the predominant mode of failure in rectal cancer (5). Thus, alternative treatment modalities are necessary. This article explores the issues, recent advances and paradigm shifts in the management of LARC, and emphasizes the need for a personalized treatment plan based on tumor stage, location, gene expression and quality of life. We hope that this will create a profound awareness about the controversies surrounding LARC management and assist in formulating more effective treatment strategies.
Standards of care: LCRT vs. SCRT
A pioneering trial from Germany in 2004 signalled the advent of preoperative chemoradiotherapy (CRT) as the standard of care for LARC. In the German CAO/ARO/AIO-94 trial, neoadjuvant treatment showed significant improvement over adjuvant treatment in terms of local recurrence (LR) (6% vs. 13%, P=0.006) and toxicity (27% vs. 40%, P=0.001). Neoadjuvant CRT is better tolerated than postoperative CRT, and provides the capability of achieving curative resection after downstaging and improving sphincter preservation rates. The German trial established preoperative CRT as the preferred concurrent regimen, after which the use of postoperative CRT declined rapidly (7). Around this time, randomized controlled trials demonstrated that neoadjuvant SCRT also improved LR as compared to surgery alone; however, there was little impact on disease-free survival (DFS) or OS (8).
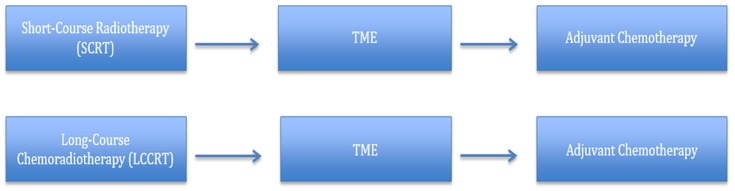
Both neoadjuvant LCRT [45.0–50.4 Gy in 25 fractions with concurrent 5-fluorouracil (5-FU) chemotherapy] and SCRT (25.0 Gy in 5 fractions) are considered standards of care in the treatment of LARC (Figure 1). Both SCRT and LCRT reduce the risk of pelvic recurrence by approximately 50% and have equal ability to reduce local recurrence. Advocates of SCRT emphasize the regimen’s simplicity, excellent local control, high rates of compliance (90%) and patient convenience. LCRT advocates highlight the excellent downsizing of the tumor and superior sphincter preservation rates (8). Randomized controlled trials comparing SCRT and LCRT to elucidate their benefits, assist in clinical practice, and guide future research directions showed essentially similar oncologic outcomes. No significant difference was established between treatment arms; however, SCRT was advocated if lower cost, better compliance and convenience are sought (9-11).
1.
Standard therapies for locally advanced rectal cancer. TME, total mesorectal excision.
These randomized controlled trials aimed to demonstrate the relative merits of SCRT and LCRT; nevertheless, it remains unclear which neoadjuvant treatment is better. The Berlin Rectal Cancer trial aims to settle the optimal course of neoadjuvant CRT; the results are pending (12). Questions abound regarding the optimal preoperative approach. Regional differences are noted in the treatment approach to LARC. Although SCRT has been adopted by European countries as the standard approach, the majority of US and Korean surgeons prefer the standard of care established by the German trial. LCRT remains the preferred treatment in most centers, especially in averting the significant morbidity associated with local recurrence and in dealing with high risk factors, such as distally located tumors and CRM involvement (4,13).
Future perspectives and novel strategies in rectal cancer treatment
Induction chemotherapy vs. consolidation chemotherapy: Strategies for chemotherapy intensification
The perennial problem of distant recurrence in LARC catalyzed the emergence of novel strategies to optimize chemotherapy to control systemic disease, keep local recurrence rates below 10%, and translate into better oncologic outcomes. The NSABP R-04, PETACC-6, STAR-01, ACCORD 12 and CHRONICLE trials, which explored the efficacy of adding oxaliplatin in the neoadjuvant and adjuvant settings, consistently demonstrated alarmingly high toxicity rates, suggesting the futility of this strategy (14-19). In contrast, the addition of oxaliplatin to the neoadjuvant regimen in the German CAO/ARO/AIO-04 trial was well-tolerated and resulted in increased compliance and pathological complete response (pCR) rates (20). Similarly, the ADORE trial showed that the addition of oxaliplatin in the adjuvant setting resulted in significant improvement in terms of 3-year DFS [hazard ratio (HR), 0.66; 95% confidence interval (95% CI), 0.43–0.99; P=0.47] and OS (HR, 0.46; 95% CI, 0.22–0.97; P=0.036), indicating that oxaliplatin might be beneficial in patients who are less responsive to fluoropyrimidine-based CRT; however, longer follow-up data are needed to confirm the trial’s findings (21).
The use of a more intensified neoadjuvant regimen is a promising alternative in enhancing systemic control and improving treatment compliance. Induction chemotherapy, which refers to systemic chemotherapy given before LCRT and surgery, emphasizes the potential of chemotherapy in treating micrometastases, thereby reducing the incidence of distant recurrence and improving survival. The purpose of induction chemotherapy is to reduce distant relapse by early treatment initiation, allow downstaging of the primary tumor, and identify aggressive tumors that would not respond to treatment (22). Given that it takes 16–19 weeks following CRT to initiate systemic chemotherapy, it is imperative that upfront chemotherapy be started in patients who are likely to require chemotherapy as part of their treatment (23). Theoretically, the early administration of chemotherapy would inhibit the development and dissemination of metastatic disease and reduce the incidence of distant recurrence (2). This makes induction chemotherapy particularly appealing, as it may improve long-term outcomes.
Several trials have evaluated induction chemotherapy followed by surgery with encouraging results (Table 1). In 2002, a study by Chau et al. showed that chemotherapy as an introduction to CRT can be administered with a negligible risk of disease progression and resultant amelioration of tumor symptoms, such as improvement in bowel habits, diminished rectal bleeding, and reduced pelvic pain (24). The encouraging initial results corroborated the subsequent phase II single-arm EXPERT trial by Chua et al., which used capecitabine and oxaliplatin (CAPOX) for induction therapy (25). The study enrolled 105 patients with poor-risk disease. At least one of the following characteristics was required: threatened or involved CRM; low-lying T3 tumor situated at the levator ani; a tumor with >5 mm extension into the perirectal fat; T4 tumors; and T1–4N2 tumors. Of the 105 patients, 97 underwent surgery. Among those who underwent surgery, TME was performed in 95 patients, and 97% had clear resection margins. The phase II trial recorded high radiological response (RR) rates (74% after induction chemotherapy and 89% after CRT) and surpassed the number of pathological responses [20% (21/105)] needed to meet the objective of the trial. The 3-year progression-free survival (PFS) and OS were 68% (95% CI, 59%–77%) and 83% (95% CI, 76%–91%), respectively. The 3-year relapse-free survival (RFS) for patients who underwent complete resection was 74% (95% CI, 65%–83%). Given that oxaliplatin is associated with grade 3–4 toxic effects, the authors deduced that administration of combination chemotherapy before standard CRT could be a more tolerable means of delivering oxaliplatin systematically ( 25). The results of the EXPERT trial were replicated in a Danish study, which revealed OS (65%) and DFS (63%) rates similar to other trials (26). The encouraging results of the phase II EXPERT trial led to the EXPERT-C trial, which evaluated induction CAPOX and preoperative CRT with or without cetuximab followed by TME. The incorporation of cetuximab fell short in improving the primary endpoint of complete response (CR) (pCR or, in patients not undergoing surgery, radiologic CR) [9% vs. 11%; P>0.99; odds ratio (OR), 1.22] or PFS (HR, 0.65; P=0.363). In contrast, cetuximab proved to be beneficial in patients with KRAS/BRAF wild-type cancer, leading to significant improvement in RR [71% (CAPOX + cetuximab)vs. 51% (CAPOX alone)]. Cetuximab also enhanced OS (HR, 0.27; P=0.034) (22).
1.
Trials of induction chemotherapy followed by CRT in LARC
| Trial | Study design | Number | Induction
chemotherapy |
CRT | Adjuvant chemotherapy | pCR | Outcomes |
| Chau et al. (24) | Single arm | 36 | 5-FU +
mitomycin C |
5-FU + RT | 5-FU + mitomycin C | 2.7% | After chemotherapy:
-Radiologic tumor response: 27.8% -Symptomatic response: 65% After CRT: -Tumor regression: 80.6% -R0 resection: 82% -Pathological T, N downstaging stage: 73.4% -Toxicity: 25% -Mortality: 2.7% -Morbidity: 13.8% |
| EXPERT Chua et al. (25) | Single arm | 105 | CAPOX | Capecitabine + RT | Capecitabine | 20% | After chemotherapy:
-Radiologic response: 74% After CRT: -Radiologic response: 89% -Long-term outcomes: 3-year DFS 68% 3-year OS 83% 3-year PFS 74% |
| EXPERT-C Dewdney et al. (22) | Randomized controlled | 165 | CAPOX | Capecitabine + RT | CAPOX | 7% | After chemotherapy:
-Radiologic response: 51% After CRT: -Radiologic response: 75% |
| CAPOX + C | Capecitabine + RT | CAPOX + C | 11% | After chemotherapy:
-Radiologic response; 71% After CRT: -Radiologic response: 93% -OS (HR, 0.27; P=0.034) Favoring CPOX + C -PFS: no difference |
|||
| Schou et al. (26) | Single arm | 85 | CAPOX | Capecitabine + RT | – | 23% | R0 resection: 94%
T downstaging: 69% DFS: 63%(95% CI: 52.2%–73.7%) |
| GCR-3 (27) | Randomized controlled | 108 | – | CAPOX + RT | CAPOX | 13.5% | R0 resection: 87%
Downstaging: 59% Toxicity: 54% DFS: 64% OS: 78% LR: 2% DM: 21% |
| Table 1 (continued) | |||||||
Toxicity and patient refusal are the two main reasons for poor compliance with systemic treatment after CRT and surgery. Moreover, persistent toxicities or a decrease in performance status after preoperative CRT and surgery jeopardizes compliance to adjuvant chemotherapy, consequently increasing the risk of relapse and compromising survival (28). The use of induction chemotherapy is a strategy to address this issue, allowing full dose chemotherapy to be delivered earlier and enabling better compliance to treatment. The Spanish GCR-3 study, the largest prospective two-arm randomized controlled trial, recruited 108 rectal cancer patients (T3/4 and/or node-positive disease). Patients were randomly assigned to Arm A (preoperative CRT followed by surgery and postoperative adjuvant therapy), or Arm B (induction CAPOX followed by CRT and surgery). There were no statistical differences between the two arms in terms of short-term outcomes, such as pCR (13.5% vs. 14.3%), downstaging, tumor regression and R0 resection rates. Similarly, there was no significant advantage in long-term outcomes, yielding similar 5-year DFS (64% vs. 62%; P=0.85), LR (2% vs. 5%; P=0.61), and OS (78% vs. 75%) rates. The most noteworthy results of this study pertain to the secondary endpoints of toxicity and compliance. During CRT, there was no significant difference between the arms in grade 3 to 4 toxicity; however, more patients in arm A than in arm B experienced grade 3–4 toxicity (54% vs. 19%; P=0.0004) after treatment. In terms of treatment compliance, 94% completed the induction chemotherapy compared to just 57% completing adjuvant chemotherapy (27,32). Correspondingly, the CONTRE single arm study reported high treatment compliance (92%) using induction chemotherapy (8 cycles of FOLFOX6 followed by CRT and surgery). This resulted in resolution of bleeding and amelioration of obstructive symptoms in all patients, with 33% demonstrating pCR at surgery (28).
Subsequent trials evaluated the long-term outcomes of induction chemotherapy. Calvo et al. demonstrated that an induction FOLFOX4 regimen (induction FOLFOX4 followed by CRT and surgery) significantly improved tumor downstaging (63% vs. 54%; P=0.02), nodal downstaging (60% vs. 43%; P=0.002) and enhanced sphincter preservation rate (30% vs. 13%; P=0.04) as compared to conventional treatment (CRT followed by surgery); however, no improvement was seen in terms of DFS (HR, 0.83; P=0.55), distant metastasis-free survival (HR, 0.94; P=0.8), cancer specific survival (CSS) (HR, 0.70; P=0.15) and locoregional control (HR, 0.88; P=0.78) (31). A Swiss multicenter single arm phase II study evaluated the efficacy of induction CAPOX. Induction CAPOX resulted in improving pCR (23%), R0 resection (98%), sphincter preservation (84%), and tumor and nodal downstaging (65%) rates (30). The 5-year PFS and OS rates were 61% and 78%, respectively. As with previous long-term follow-up results, 8% of the patients had LR; however, the promising local control rates came at the expense of patients’ urinary, bowel, and stoma functions. Pursuant to this, the investigators stressed the need for long-term follow-up that would allow for weighing the risks and benefits of targeted multimodality treatments for LARC, specifically the inclusion of patient-reported outcomes and predictors of long-term toxicity in future trials (33).
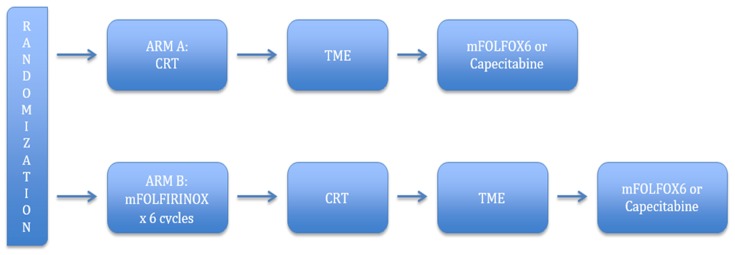
The above-mentioned trials indicate that there exists a potential benefit of systemic treatment prior to CRT and surgery in patients with LARC, necessitating further investigations in patients who would otherwise receive postoperative chemotherapy (23). The trials support that induction chemotherapy can lead to better compliance, lower toxicity, and increased response rates without compromising DFS and OS (27). Moreover, they opened a potential avenue of research, such as the incorporation of targeted anti-EGFR agents in the induction regimen rather than simultaneous with CRT (13,34). Notwithstanding the number of investigations, the benefits of induction chemotherapy remain unclear, due to small sample sizes and the use of single arm trials, with randomized studies showing no clear advantages in terms of long-term outcomes (2). At present, there is an ongoing phase III trial (PRODIGE III) that randomly assigns patients to receive either induction chemotherapy with FOLFIRINOX followed by preoperative CRT and TME (experimental arm), or preoperative CRT and TME alone (control arm). This trial should lead to better understanding of induction therapy and assist in formulating a treatment paradigm (35) (Figure 2).
2.
PRODIGE III trial. Adapted from www.clinicaltrials.gov, NCT 01804790. CRT, chemoradiotherapy; TME, total mesorectal excision.
Induction chemotherapy has become a welcome addition to the recent investigations regarding the benefits of chemotherapy intensification in LARC treatment. This presents an opportunity to explore additional strategies which would exploit the interval between neoadjuvant treatment and surgery, optimize the use of systemic chemotherapy, and allow time for tumor response to develop. Another approach is the use of consolidation chemotherapy, which is the incorporation of aggressive chemotherapy cycles between SCRT/LCRT and surgery (Table 2).
2.
Trials on consolidation chemotherapy for treatment of LARC
| Study | Study design | Number | CRT | Consolidation chemotherapy | Outcomes |
| LARC, locally advanced rectal cancer; CRT, chemoradiotherapy; 5-FU, 5-fluorouracil; RT, radiotherapy; pCR, pathological complete response; SUVmax, maximum standardized uptake value. | |||||
| Garcia-Aguilar
et al. (36) |
Non-randomized | 259 | 5-FU + RT | – | pCR: 18% |
| 5-FU + RT | 2 cycles of mFOLFOX6 | pCR: 25% | |||
| 5-FU + RT | 4 cycles of mFOLFOX6 | pCR: 30% | |||
| 5-FU + RT | 6 cycles of mFOLFOX6 | pCR: 38% | |||
| Bujko et al. (37) | Randomized controlled | 515 | RT | 6 cycles of FOLFOX | R0 resection: 77%
pCR: 16% Toxicity: 75% |
| 5-FU + oxaliplatin + RT | – | R0 resection: 11%
pCR: 12% Toxicity: 83% |
|||
| Habr Gama
et al. (38) |
Non-randomized | 99 | 5-FU + RT | – | pCR: 23%
SUVmax (0–6 weeks): 63% SUVmax (0–12 weeks): 57% |
| 12 | 5-FU + RT | 6 cycles of FOLFOX6 | pCR: 66%
SUVmax (0–6 weeks): 88% SUVmax (0–12 weeks): 90% |
||
The Lyons R90-01 trial in 1999 established the current reference interval of 6–8 weeks between completion of preoperative CRT and surgical resection (39). The results of the 17-year follow-up of the trial revealed that increasing the interval after radiotherapy has a significant influence in increasing sphincter preservation rates for distal cancer (40). Recent studies have suggested that tumor response is time-dependent, with complete tumor regression taking months before becoming evident (41,42). Likewise, an increased interval between neoadjuvant treatment and surgery is correlated with decreased recurrence, higher rates of pCR, and improved DFS (43). Taking advantage of the “resting interval” between CRT and surgery, Garcia-Aguilar et al. designed a strategy wherein mFOLFOX6 was delivered after CRT. This multi-institutional study was composed of four consecutive study groups. The 1st group was composed of patients who underwent conventional therapy (CRT followed by surgery), while the second, third and fourth groups received 2, 4 and 6 cycles, respectively, of mFOLFOX6 between CRT and TME. Eighteen percent of patients in Group 1, 25% in Group 2, 30% in Group 3 and 38% in Group 4 achieved pCR. The incorporation of chemotherapy within this “resting interval” resulted in higher pCR rates after radical surgery, with increasing pCR rates with additional chemotherapy cycles and time between CRT and surgery (36). This means that consolidation or intensified chemotherapy during the “resting interval” may increase the pCR rate, leading to better oncologic outcomes. From a surgical and oncological perspective, this strategy was deemed safe and effective, without any noted increase in tumor progression, complications, or technical difficulty during surgery (36). Habr-Gama et al. compared standard CRT to extended CRT with consolidation chemotherapy, using FDG-PET/CT to predict and assess tumor response in patients with rectal cancer. Their data suggested that consolidation chemotherapy could lead to a reduction in tumor metabolism compared to standard CRT. Patients treated with the extended regimen were more likely to achieve pCR or complete clinical response (cCR) (23% vs. 66%; P=0.0004). The maximum standardized uptake value (SUVmax) variations between 0 and 6 weeks (88% vs. 63%; P=0.001) and between 0 and 12 weeks (90% vs. 57%; P<0.001) were greater in the consolidation group. Thus, extended CRT with consolidation chemotherapy resulted in greater reduction in tumor metabolism and less frequent tumor repopulation between 6 and 12 weeks from CRT completion (38).
Recently, a Polish phase III randomized trial compared oxaliplatin-based LCRT versus 5 × 5 Gy and consolidation chemotherapy. There were no significant differences in R0 resection (77% vs. 71%; P=0.07), CR (16% vs. 12%; P=0.17), DFS (53% vs. 52%; P=0.85), and distant metastasis (30% vs. 27%; P=0.26); nevertheless, longer OS and lower toxicity were observed in the consolidation therapy group. Despite not achieving the primary endpoint of an improved R0 resection rate, the enhanced OS and reduced acute toxicity favored the use of consolidation chemotherapy. Moreover, the delivery of a shorter course of radiotherapy with consolidation chemotherapy is more economical and convenient than LCRT (37).
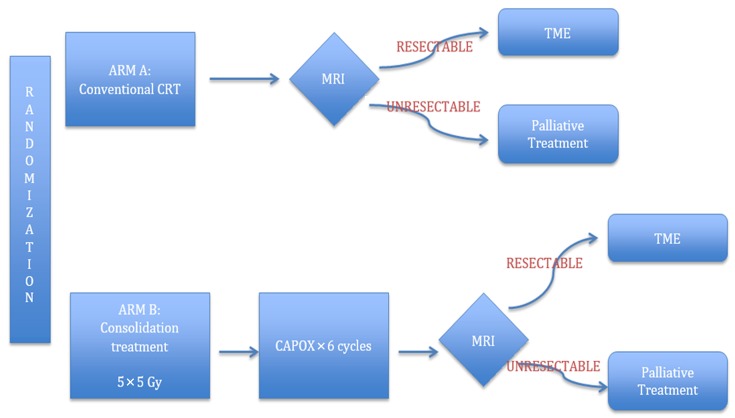
The groundbreaking MERCURY trial allowed for the identification of patients with a high risk of local and systemic relapse using MRI to facilitate patient stratification, identify prognostic factors, and optimize use of preoperative therapy (6). For high-risk tumors on MRI, which are termed “ugly” or “advanced,” CRT is the preferred regimen to improve local control and CSS; however, some patients may be too frail for CRT (44). Previous trials have supported the idea that SCRT can lead to significant downstaging. The evidence of tumor downstaging with SCRT and the contention that neoadjuvant chemotherapy is more effective than adjuvant chemotherapy formed the foundation for the Radiotherapy and Preoperative Induction therapy followed by Dedicated Operation (RAPIDO) trial (Figure 3). It aims to compare the standard CRT regimen consisting of preoperative CRT (1.8 Gy × 25 or 2 Gy × 25 with capecitabine) followed by TME and adjuvant chemotherapy to a consolidation regimen consisting of SCRT (5 Gy × 5) followed by 6 cycles of chemotherapy (CAPOX) before surgery. The primary endpoint is 3-year DFS, while the secondary endpoints include OS, local control, toxicity profile, pCR and treatment completion rate (44,45). In our opinion, the RAPIDO trial is a promising treatment paradigm for high-risk rectal cancer. SCRT would promote downstaging and locoregional control, while intensified chemotherapy would be effective in controlling systemic metastasis and improving DFS, taking full advantage of the “resting interval” for tumor regression and downstaging before surgery. If the ongoing RAPIDO trial shows improved survival and local control, it would dispute LCRT as the established treatment for LARC. In the meantime, consolidation chemotherapy may be considered to reduce cost and waiting time for radiotherapy (37).
3.
RAPIDO trial. Adapted from Nilsson et al. (44). MRI, magnetic resonance imaging; TME, total mesorectal excision.
As the conventional strategy of CRT followed by surgery reaches an efficacy plateau, chemotherapy intensification, in the form of induction and consolidation chemotherapy, has grown in relevance. This is particularly beneficial for poor-risk rectal cancer, which is characterized by a high risk of relapse and treatment failure. Both strategies may allow for better treatment compliance and assist optimal delivery of chemotherapy. After an extensive review of the literature, we stand by our experience that cost and patient tolerability should be balanced with outcome. Notwithstanding the promising results of the aforementioned trials, weighing the risks and benefits is of paramount importance, especially when dealing with long-term patient-reported outcomes as seen in the Swiss multicenter study (30). Clearly, there is still a need to explore significant predictors and assess the risk/benefit ratio of both strategies, taking into account both long-term toxicity and efficacy. Meanwhile, a multidisciplinary approach should be employed in determining the most appropriate strategy to use, taking into consideration close monitoring of the disease using advanced technology and imaging studies.
Neoadjuvant chemotherapy alone: How to avoid sequelae of radiation?
The necessity of trimodality therapy has been called into question because of improved local control outcomes and decreased local recurrence. Experts have argued that LARC patients, especially if they are TME candidates, do not require such an intensive treatment approach (2). Furthermore, the long-term consequences of pelvic irradiation on the bowel, bladder, fertility, sexual function and bone marrow reserve can be significant (4). It can be argued that radiotherapy can be eliminated in certain populations, particularly in patients not requiring abdominoperineal resection. Numerous studies have explored the possibility of eliminating radiotherapy in LARC treatment; however, no certain conclusions can be reached because of the small number of patients in these trials (2) (Table 3).
3.
Trials on neoadjuvant chemotherapy alone before TME in LARC
| Study | Design | Number | Neoadjuvant CRT | Downstaging | Outcomes |
| TME, total mesorectal excision; LARC, locally advanced rectal cancer; CRT, chemoradiotherapy; LR, local recurrence; DFS, disease-free survival; pCR, pathological complete response; RFS, relapse-free survival; 5-FU, 5-fluorouracil; OS, overall survival. | |||||
| MSKCC
(Schrag et al.) (46) |
Single arm | 32 | FOLFOX + bevacizumab | 25% | R0 resections: 100%
4-year LR: 0% 4-year DFS: 84% |
| GEMCAD 0801
(Fernandez-Martos et al.) (47) |
Single arm | 46 | CAPOX + bevacizumab | 48% | R0 resections: 100%
Overall response rate: 78% pCR: 20% Anastomotic leakage: 13% 24-month DFS: 75% 2-year LR: 2% recent updated report 41-month DFS: 61% 41-month RFS: 76% |
| NSOG-03
(Uehara et al.) (48) |
Single arm | 32 | CAPOX + bevacizumab | R0 resection: 90%
Post-operative complication: 43% pCR: 13% Treatment compliance rate: 84% Good tumorregression: 37% |
|
| Corona 1 (Kamiya
et al.) (49) |
Single arm | 41 | CAPOX | Tumor: 52.5%
Node: 56.7% |
Complication rate: 15%
pCR: 12.2% |
| Ishii et al. (50) | Single arm | 26 | Irinotecan + 5-FU + leucoverin | 57.6% | pCR:3.8%
5-year RFS: 74% 5-year OS: 84% |
Schrag et al. conducted a pilot study evaluating preoperative infused FOLFOX/bevacizumab with selective use of CRT, meaning that CRT would only be used if there was noted progression after chemotherapy. Bevacizumab was included based on its superiority in metastasis and proven benefits in adjuvant colon cancer treatment. The study was able to satisfy its primary endpoint, achieving 100% R0 resections. pCR was achieved in 25% of patients. Compliance with treatment was high at 93.8%. The 4-year LR rate and DFS rates were 0% and 84%, respectively. Based on these data, neoadjuvant chemotherapy and selective radiation seem to yield favorable outcomes (46). Likewise, the GEMCAD 0801 phase II multicenter trial showed encouraging results with the CAPOX + bevacizumab regimen, with an overall response rate of 78%, pCR of 20%, and T downstaging of 48%; however, the anastomotic leak rate of 13% was higher than expected, prompting the investigators to discourage further investigations using this regimen (47).
Preliminary studies regarding neoadjuvant chemotherapy alone yielded encouraging results; however, all of these studies only involved patients with low disease burden. In Japan, several studies explored the efficacy of neoadjuvant chemotherapy alone in poor-risk LARC patients. Uehara et al. conducted a phase II trial to evaluate neoadjuvant chemotherapy consisting of CAPOX and bevacizumab without radiotherapy. The regimen yielded satisfactory results, achieving an 84.4% completion rate and a 13.3% pCR rate; however, the study fell short of attaining acceptable toxicity complication rates, which made the investigators reconsider the necessity of bevacizumab (48). As a follow-up, they conducted the CORONA 1 prospective study, which aimed to assess the safety and efficacy of CAPOX alone. N downstaging (cN + to yN0) was seen in 56.7% of patients, while T downstaging was observed in 52.5%. pCR was observed in 12.2% of patients and tumor regression in 31.7%. The omission of bevacizumab led to a marked reduction in the rate of anastomotic leakage (25% to 12%). The pCR and tumor regression rates were equivalent in both trials. Notwithstanding the satisfactory pathological response to the CAPOX regimen, the rate of N downstaging was lower than the regimen including bevacizumab. The authors concluded that, although the preoperative CAPOX regimen showed a satisfactory response rate, the overall downstaging was inadequate. The use of preoperative CRT is still needed to achieve complete tumor regression for curative resection. The addition of bevacizumab might be beneficial in patients with massive lymph node metastasis; however, additional trials are needed to confirm its preoperative use (49).
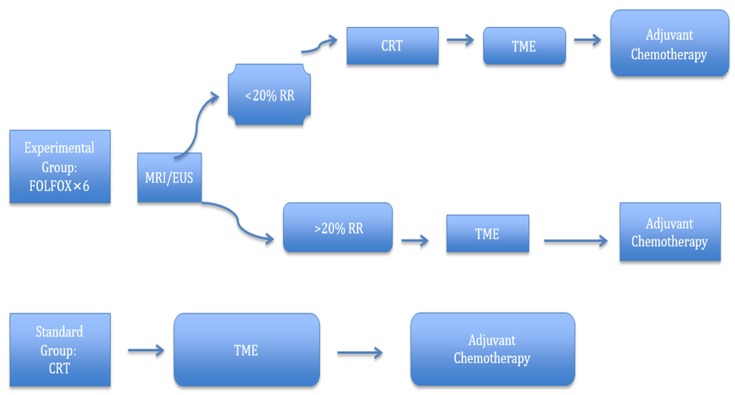
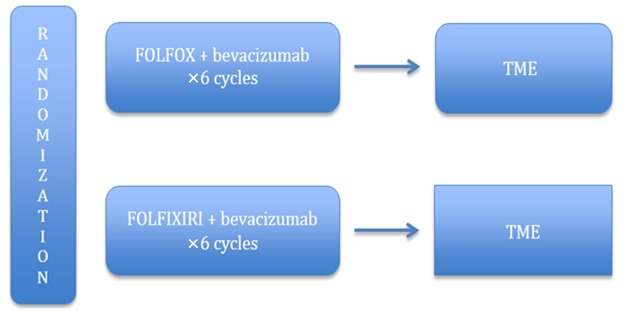
Neoadjuvant chemotherapy without radiotherapy treatment is founded on the premise that the toxicity associated with radiation can be avoided without sacrificing local control, and distal control can be achieved by the early introduction of systemic therapy. Encouraged by the promising results of the aforementioned trials, a large randomized phase III study (PROSPECT trial) comparing preoperative CRT with neoadjuvant FOLFOX is being conducted in the United States (Figure 4). This study aims to ascertain whether neoadjuvant chemotherapy followed by selective CRT can increase R0 resection rates and improve long-term oncologic outcomes compared to conventional LCRT. Moreover, the trial portends a treatment algorithm, which can avoid unnecessary radiation and toxicity, especially for low-risk LARC patients (49,51). Another phase II trial (BACCHUS trial) comparing neoadjuvant FOLFOX+bevacizumab with FOLFOXILI (oxaliplatin + irinotecan + 5-FU + leucoverin) + bevacizumab is also ongoing in the UK (Figure 5). Unlike the PROSPECT trial, the purpose of the BACCHUS study is to compare the feasibility and efficacy of two combinations of anticancer drugs before surgery, which could subsequently challenge the present culture of using routine radiotherapy for LARC (52). Once completed, these trials will play a crucial role in dealing with distant metastasis and defining the ideal treatment.
4.
PROSPECT trial. Adapted from www.clinicaltrials.gov, NCR 01515787. MRI, magnetic resonance imaging; EUS, endoscopic ultrasound; RR, radiological response; CRT, chemoradiotherapy; TME, total mesorectal excision.
5.
BACCHUS trial. Adapted from www.clinicaltrials.gov, NCT01650428. TME, total mesorectal excision.
Paradigm shift toward a simplistic and tailored approach to LARC
Developments during the last decade shaped multimodality treatments for patients with LARC. TME has been a mainstay of treatment for LARC since being introduced in the 1980s. Neoadjuvant CRT has been shown to result in tumor downstaging, leading to tumor reduction and eradication of viable tumors cells and lymph node metastases in 10%−30% of patients (52). Patients with pCR have good oncologic outcomes, leading experts to cast doubt on the value of TME in this subgroup. Selective nonoperative management (NOM) for patients who experience cCR/pCR to CRT was started at the University of Sao Paolo. In the initial report, Habr-Gama et al. described the outcomes of 265 patients with adenocarcinoma of the distal rectum treated with standard CRT (5,040 cGy delivered at 180 cGy/d for 5 days per week for 6 weeks + 5-FU + folinic acid) and assessed for response 8 weeks after CRT completion using clinical, endoscopic, and radiological parameters. Over one quarter (26.5%) of treated patients achieved cCR and were followed-up after CRT (observation group), while 8.3% showed incomplete clinical response and underwent resection (resection group). The 5-year OS (100% vs. 88%) and DFS (92% vs. 83%) rates were superior in the observation group compared to the resection group. Therefore, CRT may lead to substantial increases in cCR and pCR in distal rectal cancer. The attainment of Stage 0 disease after CRT is associated with excellent long-term results and may warrant close observation instead of surgery without any oncologic compromise (53).
Identification of the cCR and pCR parameters has been the major barrier in this strategy. There are no standardized patient criteria or management protocols. A recent systematic review, including 15 studies and 920 patients, revealed that the OS and DFS of the NOM group were 91.7% and 82.7%, respectively. Therefore, NOM may be a possible alternative for patients who achieve pCR after neoadjuvant CRT as long as they undergo close surveillance; however, before this paradigm can be widely adopted, standardized definitions and management protocols should be developed (54).
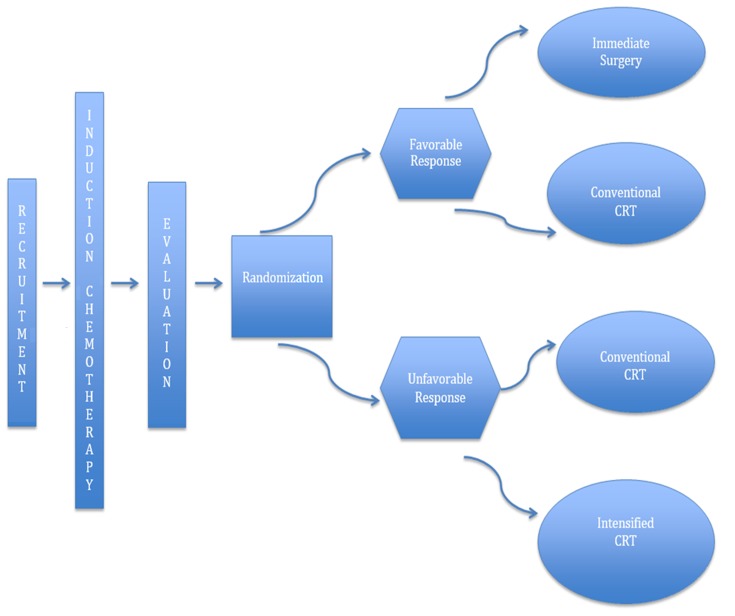
Recently, calls for individualized tailored treatment strategies have gained momentum. Subgroups of patients with LARC respond in varying degrees to CRT and are associated with different levels of recurrence and survival. Tumor response, as evidenced by pCR/cCR after CRT, has emerged as an important prognosticator, associated with improved survival and decreased local and distant recurrence. Stratification based on tumor response has led experts to doubt the feasibility of an intensive multimodality approach (5,55). The GRECCAR4 trial was conducted to tailor treatment based on tumor response to induction chemotherapy, allowing identification of patients in whom treatment regimens could be intensified or reduced (Figure 6). The trial enrolled 206 patients from 16 French centers. The FOLIFIRINOX regimen was used for induction chemotherapy. The standard CRT regimen consisted of 50 Gy and capecitabine, while the intensified CRT regimen consisted of 60 Gy and capecitabine. Good responders (≥75% tumor reduction) to induction chemotherapy were randomly assigned to immediate surgery (Arm A) or standard CRT (Arm B), and poor responders (<75% tumor reduction) were randomly assigned to standard CRT (Arm C) or intensified CRT (Arm D). They found that tumors sensitive to chemotherapy can be managed with immediate surgery without neoadjuvant CRT, while the high pCR (60%) rate in Arm B suggests that there is room for a less aggressive surgical approach. In contrast, the R0 resection rates (Arm C: 88%; Arm D: 83%) of the poor responders were comparable to those described in the literature. Despite the small population size, the results of the GRECCAR4 trial are adequately convincing to warrant a larger trial (56). The GRECCAR4 trial is a harbinger of a less monolithic and more individualized approach to LARC treatment. The study set in motion tailored LARC treatment based on tumor response, which subsequently resulted in encouraging R0 resection rates. More importantly, it fostered discussion regarding alternative treatment strategies, especially for poor-risk LARC patients.
6.
GRECCAR4 trial. Adapted from Rouanet et al. (54). CRT, chemoradiotherapy.
LARC is a complex disease that demands complex answers. The aforementioned trials have aimed to challenge the pervading culture in LARC: that conventional CRT is an adequate treatment for T3–T4 “ugly” high-risk rectal cancer characterized by positive EMVI, the presence of lateral lymph nodes, and poor prognosis (44,57). The use of chemotherapeutic agents in LARC treatment continues to evolve. Neoadjuvant systemic chemotherapy alone is a promising option to avoid the complications of radiation treatment. Moreover, shifting chemotherapy use to the preoperative setting is another alternative. The use of induction chemotherapy and consolidation chemotherapy are aimed to improve long-term oncologic outcomes and lower the risk of metastatic relapse. In recent years, the concept of NOM in patients who achieved pCR has gained ground, allowing patients to avoid unnecessary morbidity due to surgery. Recent trends have advocated for a well-designed treatment plan. Results from the RAPIDO, PRODIGE, PROSPECT and BACCHUS trials are all awaited with anticipation. Once completed, these trials will assist in defining the ideal rectal cancer regimen. Ultimately, the goal underscores the need for a more individualized and streamlined treatment plan for every patient. Hence, it is imperative that a new upgraded risk-adapted treatment protocol be employed to prevent treatment failure and enhance oncological results.
Conclusions
Despite the rapid progress made during the last decade, distant recurrence remains the Achilles heel in LARC treatment. Several strategies and paradigms have been employed to circumvent metastatic spread. The management of LARC does not follow a “one size fits all” approach. These trials indicate that a more individualized approach is warranted, with the treatment adapted according to location, stage, and response. Further advances may allow for more accurate treatment and prediction of outcomes. The recent trend has been toward a more simplified and tailored approach, questioning the necessity of surgery and optimizing the use of chemotherapy. In the meantime, additional studies, as well as molecular profiling, need to be performed in order to help improve the treatment of patients with LARC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global Cancer Statistics 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Salem ME, Hartley M, Unger K, et al. Neoadjuvant combined-modality therapy for locally advanced rectal cancer and its future direction. Oncology (Williston Park) 2016;30:546–62. [PubMed] [Google Scholar]
- 3.Cameron JL, Cameron AM. Current Surgical Therapy (11th edition). Amsterdam: Elsevier, 2013.
- 4.Lee M, Gibbs P, Wong R. Multidisciplinary management of locally advanced rectal cancer — An evolving landscape? Clin Colorectal Cancer. 2015;14:251–61. doi: 10.1016/j.clcc.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Smith JJ, Garcia-Aguilar J. Advances and challenges in treatment of locally advanced rectal cancer. J Clin Oncol. 2015;33:1797–808. doi: 10.1200/JCO.2014.60.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor FG, Quirke P, Heald RJ et al, et al. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg. 2011;253:711–9. doi: 10.1097/SLA.0b013e31820b8d52. [DOI] [PubMed] [Google Scholar]
- 7.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–33. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 8.Ngan SY. Preoperative treatment of locally advanced rectal cancer: assets and drawbacks of short course and long course in clinical practice. Semin Radiat Oncol. 2016;26:186–92. doi: 10.1016/j.semradonc.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Bujko K, Nowacki MP, Nasierowska‐Guttmejer A, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215–23. doi: 10.1002/bjs.5506. [DOI] [PubMed] [Google Scholar]
- 10.Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group Trial 01.04. J Clin Oncol. 2012;30:3827–33. doi: 10.1200/JCO.2012.42.9597. [DOI] [PubMed] [Google Scholar]
- 11.Latkauskas T, Pauzas H, Kairevice L, et al. Preoperative conventional chemoradiotherapy versus short-course radiotherapy with delayed surgery for rectal cancer: results of a randomized controlled trial. BMC Cancer. 2016;16:927. doi: 10.1186/s12885-016-2959-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel R, Burock S, Wernecke KD, et al. Preoperative short-course radiotherapy versus combined radiochemotherapy in locally advanced rectal cancer: a multi-centre prospectively randomised study of the Berlin Cancer Society. BMC Cancer. 2009;9:50. doi: 10.1186/1471-2407-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rödel C, Hofheinz R, Fokas E. Rectal cancer: Neoadjuvant chemotherapy. Best Pract Res Clin Gastroenterol. 2016;30:629–39. doi: 10.1016/j.bpg.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Allegra CJ, Yothers G, O’Connell MJ, et al. Neoadjuvant 5-FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: A phase III randomized clinical trial. J Natl Cancer Inst 2015;107. pii: djv248.
- 15.Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: Pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773–80. doi: 10.1200/JCO.2010.34.4911. [DOI] [PubMed] [Google Scholar]
- 16.Gérard JP, Azria D, Gourgou-Bourgade S, et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol. 2012;30:4558–65. doi: 10.1200/JCO.2012.42.8771. [DOI] [PubMed] [Google Scholar]
- 17.O’Connell MJ, Colangelo LH, Beart RW, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project Trial R-04. J Clin Oncol. 2014;32:1927–34. doi: 10.1200/JCO.2013.53.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.An X, Lin X, Wang FH, et al. Short term results of neoadjuvant chemoradiotherapy with fluoropyrimidine alone or in combination with oxaliplatin in locally advanced rectal cancer: a meta-analysis. Eur J Cancer. 2013;49:843–51. doi: 10.1016/j.ejca.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 19.Glynne-Jones R, Counsell N, Quirke P, et al. Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol. 2014;25:1356–62. doi: 10.1093/annonc/mdu147. [DOI] [PubMed] [Google Scholar]
- 20.Rödel C, Graeven U, Fietkau R, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16:979–89. doi: 10.1016/S1470-2045(15)00159-X. [DOI] [PubMed] [Google Scholar]
- 21.Hong YS, Nam BH, Kim KP, et al. Oxaliplatin, fluorouracil, and leucoverin versus fluorouracil and leucoverin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2014;15:1245–53. doi: 10.1016/S1470-2045(14)70377-8. [DOI] [PubMed] [Google Scholar]
- 22.Dewdney A, Cunningham D, Tabernero J, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk cancer (EXPERT-C) J Clin Oncol. 2012;30:1620–7. doi: 10.1200/JCO.2011.39.6036. [DOI] [PubMed] [Google Scholar]
- 23.Dewdney A, Cunningham D, Chau I, et al. Selecting patients with locally advanced rectal cancer for neoadjuvant treatment strategies. Oncologist. 2013;18:833–42. doi: 10.1634/theoncologist.2013-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chau I, Allen M, Cunningham D, et al. Neoadjuvant systemic fluorouracil and mitomycin C prior to synchronous chemoradiation is an effective strategy in locally advanced rectal cancer. Br J Cancer. 2003;88:1017–24. doi: 10.1038/sj.bjc.6600822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chua YJ, Barbachano Y, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol. 2010;11:241–8. doi: 10.1016/S1470-2045(09)70381-X. [DOI] [PubMed] [Google Scholar]
- 26.Schou JV, Larsen FO, Rasch L, et al. Induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiotherapy before total mesorectal excision in patients with locally advanced rectal cancer. Ann Oncol. 2012;23:2627–33. doi: 10.1093/annonc/mds056. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Martos C, Garcia-Albeniz X, Pericay C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial. Ann Oncol. 2015;26:1722–8. doi: 10.1093/annonc/mdv223. [DOI] [PubMed] [Google Scholar]
- 28.Perez K, Safran H, Sikov W, et al. Complete neoadjuvant treatment for rectal cancer: The Brown University Oncology Group CONTRE Study. Am J Clin Oncol. 2017;40:283–7. doi: 10.1097/COC.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 29.Maréchal R, Vos B, Polus M, et al. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: a randomized multicentric phase II study. Ann Oncol. 2012;23:1525–30. doi: 10.1093/annonc/mdr473. [DOI] [PubMed] [Google Scholar]
- 30.Koeberle D, Burkhard R, von Moos R, et al. Phase II study of capacitabine and oxaliplatin given prior to and concurrently with preoperative pelvic radiotherapy in patients with locally advanced rectal cancer. Br J Cancer. 2008;98:1204–9. doi: 10.1038/sj.bjc.6604297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calvo FA, Sole CV, Serrano J, et al. Preoperative chemoradiation with or without induction oxaliplatin plus 5-fluorouracil in locally advanced rectal cancer. Long-term outcome analysis. Strahlenther Onkol. 2014;190:149–57. doi: 10.1007/s00066-013-0469-0. [DOI] [PubMed] [Google Scholar]
- 32.Fernández-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol. 2010;28:859–65. doi: 10.1200/JCO.2009.25.8541. [DOI] [PubMed] [Google Scholar]
- 33.Hess V, Winterhalder R, von Moos R, et al. Capecitabine and oxaliplatin prior and concurrent to preoperative pelvic radiotherapy in patients with locally advanced rectal cancer: long-term outcome. Clin Colorectal Cancer. 2017;16:240–45. doi: 10.1016/j.clcc.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Fornaro L, Caparello C, Vivaldi C, et al. Bevacizumab in the pre-operative treatment of locally advanced rectal cancer: a systematic review. World J Gastroenterol. 2014;20:6081–91. doi: 10.3748/wjg.v20.i20.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conroy T. Phase III study comparing preoperative chemoradiotherapy alone versus neoadjuvant chemotherapy with folfirinox regimen followed by preoperative chemoradiotherapy for patients with resectable locally advanced rectal cancer (Néofirinox). Available online: http://clinicaltrials.gov/ct2/show/NCT01804790?term=Prodige=III&rank=6
- 36.Garcia-Aguilar J, Chow OS, Smith DD, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16:957–66. doi: 10.1016/S1470-2045(15)00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bujko K, Wyrwicz L, Rutkowski A, et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol. 2016;27:834–42. doi: 10.1093/annonc/mdw062. [DOI] [PubMed] [Google Scholar]
- 38.Habr-Gama A, Perez RO, São Julião GP, et al. Consolidation chemotherapy during neoadjuvant chemoradiation (CRT) for distal rectal cancer leads to sustained decrease in tumor metabolism when compared to standard CRT regimen. Radiat Oncol. 2016;11:24. doi: 10.1186/s13014-016-0598-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on the downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17:2396. doi: 10.1200/JCO.1999.17.8.2396. [DOI] [PubMed] [Google Scholar]
- 40.Glehen O, Chapet O, Adham M, et al. Long-term results of the Lyons R90-01 randomized trial of preoperative radiotherapy with delayed surgery and its effect on sphincter-saving surgery in rectal cancer. Br J Surg. 2003;90:996–8. doi: 10.1002/bjs.4162. [DOI] [PubMed] [Google Scholar]
- 41.Habr-Gama A, Perez RO, Sabbaga J, et al. Increasing the rates of complete response to neoadjuvant chemoradiotherapy for distal rectal cancer: results of a prospective study using additional chemotherapy during the resting period. Dis Colon Rectum. 2009;52:1927–34. doi: 10.1007/DCR.0b013e3181ba14ed. [DOI] [PubMed] [Google Scholar]
- 42.Wasserberg N. Interval to surgery after neoadjuvant treatment for colorectal cancer. World J Gastroenterol. 2014;20:4256–62. doi: 10.3748/wjg.v20.i15.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tulchinsky H, Shmueli E, Figer A, et al. An Interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol. 2008;15:2661–7. doi: 10.1245/s10434-008-9892-3. [DOI] [PubMed] [Google Scholar]
- 44.Glimelius B, Tiret E, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann Oncol 2013;24 Suppl 6:vi81-8.
- 45.Nilsson PJ, van Etten B, Hospers GA, et al. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer -- the RAPIDO trial. BMC Cancer. 2013;13:279. doi: 10.1186/1471-2407-13-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schrag D, Weiser MR, Goodman KA, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32:513–8. doi: 10.1200/JCO.2013.51.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernandez-Martos C, Brown G, Estevan R, et al. Preoperative chemotherapy in patients with intermediate-risk rectal adenocarcinoma selected by high-resolution magnetic resonance imaging: the GEMCAD 0801 Phase II Multicenter Trial. Oncologist. 2014;19:1042–3. doi: 10.1634/theoncologist.2014-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uehara K, Hiramatsu K, Maeda A, et al. Neoadjuvant oxaliplatin and capecitabine and bavacizumab without radiotherapy for poor-risk rectal cancer: N-SOG 03 phase II trial. Jpn J Clin Oncol. 2013;43:964–71. doi: 10.1093/jjco/hyt115. [DOI] [PubMed] [Google Scholar]
- 49.Kamiya T, Uehara K, Nakayama G, et al. Early results of multicenter phase II trial of perioperative oxaliplatin and capecitabine without radiotherapy for high-risk rectal cancer: CORONA I study. Eur J Surg Oncol. 2016;42:829–35. doi: 10.1016/j.ejso.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 50.Ishii Y, Hasegawa H, Endo T, et al. Medium-term results of neoadjuvant systemic chemotherapy using irinotecan, 5-fluorouracil, and leucovorin in patients with locally advanced rectal cancer. Eur J Surg Oncol. 2010;36:1061–5. doi: 10.1016/j.ejso.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 51.Schrag D. PROSPECT: Chemotherapy alone/chemotherapy plus radiation therapy in treating patients with locally advanced rectal cancer undergoing surgery. Available online: https://clinicaltrials.gov/ct2/show/NCT01515787
- 52.Glynne-Jones R, Hava N, Goh V, et al. Bevacizumab and Combination Chemotherapy in rectal cancer Until Surgery (BACCHUS): a phase II, multicentre, open-label, randomised study of neoadjuvant chemotherapy alone in patients with high-risk cancer of the rectum. BMC Cancer. 2015;15:764. doi: 10.1186/s12885-015-1764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation results long-term results. Ann Surg. 2004;240:711–7. doi: 10.1097/01.sla.0000141194.27992.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sammour T, Price BA, Krause KJ, et al. Nonoperative management or " watch and wait” for rectal cancer with complete clinical response after adjuvant chemoradiotherapy: a critical appraisal. Ann Surg Oncol. 2017;24:1904–15. doi: 10.1245/s10434-017-5841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–44. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 56.Rouanet P, Rullier E, Lelong B, et al. Tailored treatment strategy for locally advanced rectal carcinoma based on the tumor response to induction chemotherapy: preliminary results of the French phase III multicenter GRECCAR4 trial. Dis Colon Rectum. 2017;60:653–63. doi: 10.1097/DCR.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 57.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines<sup>®</sup>). Rectal Cancer (Version 3. 2017). Available online: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf