Abstract
Gene therapy is an attractive approach for disease treatment. Since platelets are abundant cells circulating in blood with the distinctive abilities of storage and delivery and fundamental roles in hemostasis and immunity, they could be a unique target for gene therapy of diseases. Recent studies have demonstrated that ectopic expression of factor VIII (FVIII) in platelets under control of the platelet-specific promoter results in FVIII storage together with its carrier protein von Willebrand factor (VWF) in α-granules and the phenotypic correction of hemophilia A. Importantly, the storage and sequestration of FVIII in platelets appears to effectively restore hemostasis even in the presence of functional-blocking inhibitory antibodies. This review summarizes studies on platelet-specific gene therapy of hemophilia A as well as hemophilia B.
Keywords: hemophilia, gene therapy, FVIII, FIX, tissue-specific expression, platelet, immune tolerance
Introduction
Hemophilia A is a recessive X-linked bleeding disorder resulting from a factor VIII (FVIII) deficiency.1 Although protein replacement therapy is effective for patients with hemophilia A, it is expensive and requires frequently accessing blood vessels, which limits its universal availability.2, 3 Furthermore, up to 30% of patients with severe hemophilia A will develop inhibitory antibodies after protein infusion.4, 5, 6 These inhibitory antibodies, referred to as inhibitors, will inactivate functional FVIII activity, rendering routine protein replacement therapy useless for bleeding episodes in patients with hemophilia A.7, 8, 9 Gene therapy has the promise of treating hemophilia A but, in addition, has brought up a question about the importance of its relation to the biosynthesis of its carrier protein von Willebrand factor (VWF). This paper reviews site-specific FVIII expression in platelets, which are one of the two cell types synthesizing and storing VWF in the body, for gene therapy of hemophilia A and hemophilia B.
Platelet-Specific Gene Therapy of Hemophilia A and Hemophilia A with Inhibitors
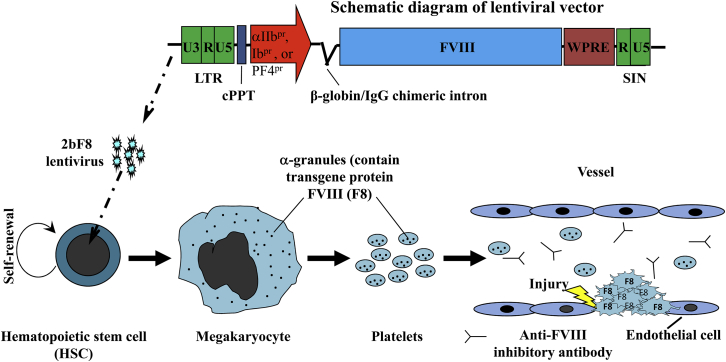
Directing FVIII expression to the cells that synthesize VWF (platelets or endothelial cells) in vivo could potentially result in the formation of an intracellular VWF/FVIII complex and enhance the stability of FVIII compared to targeting cells, which do not synthesize VWF (e.g., stromal cells or hepatocytes). Targeting FVIII expression to platelets could be especially beneficial for gene therapy of hemophilia A because FVIII will be delivered together with VWF to the site of injury. This is particularly important for hemophilia A with inhibitors because FVIII would be sequestered by platelets, avoiding inhibitor inactivation in the circulation. Furthermore, a substantial amount of FVIII may be released from platelets at hemostatic sites, where aggregated platelets become activated at sites of injury, thus circumventing the time-dependent inactivation by inhibitors and achieving improved hemostasis. Several groups have been devoting efforts to develop unique gene therapy protocols using platelets as a target to deliver therapeutics for hemophilia A treatment. Various platelet lineage-specific promoters have been utilized to direct FVIII expression to platelets, including the platelet glycoprotein (GP) IIb (αIIb) promoter, GPIb promoter, and platelet factor-4 (PF4) promoter. Both transgenesis- and lentivirus-mediated gene delivery have been used to introduce platelet-specific FVIII expression. A schematic diagram of platelet-specific gene therapy of hemophilia A is depicted in Figure 1.
Figure 1.
Schematic Diagram of Platelet-Specific Gene Therapy of Hemophilia A
Lentiviral vectors harboring FVIII expression cassette under control of a platelet-specific promoter (αIIb, Ib, or PF4 promoter) are used to transduce hematopoietic stem cells (HSCs). Transduced HSCs undergo self-renewal as well as differentiation into megakaryocytes, where FVIII transgene protein will be made and stored in α-granules, which will be shed into platelets circulating in blood. Platelet-sequestered FVIII will be protected from anti-FVIII inhibitory antibody inactivation. At the site of injury, FVIII (together with its carrier protein VWF) will be released from activated platelets, and thus time-dependent inhibitor activation may be circumvented, achieving improved hemostasis. Figure was used by permission of Q. Shi.
Proof-of-Principal of Platelet-Specific Gene Therapy of Hemophilia A Using Transgenic Mouse Models
The αIIb Promoter-Driven Model
To restrict FVIII expression to the platelet lineage, Shi and co-workers10 have developed a vector, named 2bF8, in which human B-domain-deleted FVIII expression is driven by the platelet-specific αIIb promoter. The human FVIII expression cassette used in the 2bF8 model has the complete B-domain deleted, removing residues 741 through 1468 of FVIII. There is no amino acid SQ sequence (SFSQNPPVLKRHQR) remaining in this hFVIII cassette, which was confirmed by DNA sequencing in subsequent studies and used in endothelial cell-specific FVIII expression model studies as well.11 Although it had been assumed that the SQ sequence containing the furin cleavage recognition site might be beneficial to FVIII biological activity and has been used to produce FVIII product for clinical treatment of patients with hemophilia A,12 recent studies have demonstrated that the residual furin site in SQ actually is detrimental to FVIII secretion and procoagulant activity.13, 14, 15 In addition, having furin site in SQ may reduce the VWF binding affinity and reduce FVIII stability.15
In ex vivo studies, Shi et al.10 found that FVIII expression in Dami cells, a megakaryocyte cell line, is greater when driven by the αIIb promoter compared to the cytomegalovirus (CMV) promoter. When the 2bF8 expression cassette was used to generate transgenic mice on a FVIII knockout background (2bF8tg) by embryonic stem cell (ESC)-mediated transgenesis,16 FVIII was specifically expressed in platelets and stored together with endogenous murine VWF in the α-granules of platelets. Their studies showed platelet-derived FVIII can efficiently rescue the bleeding diathesis in hemophilia A mice, and clinical efficacy can be achieved by platelet transfusion or bone marrow transplantation. Using chronic models by FVIII immunization (with adjuvant) or splenocyte transfer from immunized FVIIInull mice and an acute model by infusion of inhibitory plasma from immunized FVIIInull mice, they demonstrated that platelet-derived FVIII is therapeutically effective even in the presence of high titers of anti-FVIII inhibitors. With no detectable plasma FVIII, the level of FVIII in platelets of heterozygous 2bF8tg mice corresponded to about 1.4% of FVIII in normal mouse whole blood. Remarkably, the therapeutic benefit of this platelet-FVIII surpassed the benefit of 100% plasma FVIII in the presence of inhibitors, using a tail-clip model.16 This was the first study demonstrating the clinical efficacy of platelet-derived FVIII for treating hemophilia A in the presence of inhibitors.
Further studies by Shi et al.17 using 2bF8tg mice have demonstrated that preexisting anti-FVIII immunity does not preclude 2bF8 genetically modified therapeutic engraftment when a sufficient preconditioning regimen is employed. Importantly, the titers of inhibitors in recipients declined with time after transplantation of 2bF8tg bone marrow, and the antibody eradication time was significantly shortened compared to the control group that received bone marrow transplantation from FVIIInull mice. These results indicate that platelet-containing FVIII does not trigger a memory immune response. Instead, it may induce immune tolerance even in a primed model. Their studies demonstrated that efficient preconditioning is essential to create a bone marrow niche for engrafting 2bF8 genetically engineered hematopoietic stem cells (HSCs). Using mixed transplantation of bone marrow cells from FVIIInull and 2bF8tg mice, the studies further showed that even with only 1%–5% of FVIII-containing platelets, the bleeding phenotype was still significantly improved in hemophilia A mice with pre-existing anti-FVIII immunity.
To investigate the role of VWF in platelet gene therapy of hemophilia A, Shi et al.16 developed another transgenic mouse model by crossing the 2bF8 transgene onto a VWF and FVIII double knockout background (2bF8tg/VWF−/−). They found that the level of platelet-FVIII expression was decreased significantly, but the bleeding phenotype was still rescued in 2bF8tg/VWF−/− mice in the absence of antibodies in a tail-clip injury model. However, the clinical efficacy was abrogated in the presence of inhibitors.18 They performed a series of bone marrow transplantations to further elucidate the functional properties of platelet-VWF and plasma-VWF in platelet-specific FVIII gene therapy of hemophilia A with inhibitors. They found that platelet-VWF impacts the clinical efficacy of platelet-FVIII in restoring hemostasis in hemophilia A mice in the presence of inhibitors more than plasma-VWF. For optimal platelet-derived FVIII gene therapy of hemophilia A and hemophilia A with inhibitors, both platelet-VWF and plasma-VWF are required. Together, these studies demonstrate that VWF is critical in platelet gene therapy of hemophilia A in the presence of anti-FVIII inhibitors.
The GPIbα Promoter-Driven Model
Yarovoi and co-workers19 have developed a transgenic model in which human B-domainless (hB−) FVIII (hB−F8) expression is directed by the platelet-specific GPIbα promoter (IbF8). In their highest-expressing hB−F8 transgenic line, h38/F8null, FVIII was detected in platelet releasates with a level corresponding to about 9% of the antigen and 3% of the activity in normal mouse plasma as determined by ELISA and Coatest assays. This platelet-FVIII restored hemostasis in hemophilia A mice in a FeCl3 carotid artery injury model. The cuticular injury model and whole-blood clotting time confirmed that the hemophilic phenotype was improved in transgenic mice. Further studies by Yarovoi et al.20 demonstrated that FVIII is still stored in the α-granules of platelets even in the absence of VWF and that platelet-derived FVIII without VWF can still rescue the hemophilic phenotype in hemophilia A mice in the absence of inhibitors, and the clinical efficacy was similar to that in the presence of VWF although the level of platelet-FVIII was significantly reduced.
To compare the clinical efficacy of platelet-FVIII to plasma FVIII in the presence of anti-FVIII inhibitors, Gewirtz et al.21 infused a mixture of monoclonal antibodies into either IbF8 transgenic mice, line h38/F8null, with platelet-FVIII expression or FVIIInull mice preinfused with recombinant human FVIII (rhFVIII). Under those conditions, Gewirtz and coworkers21 found that platelet-FVIII provided slightly better clot-forming activity in the FeCl3 induced thrombosis model. There is a difference in outcome between studies conducted by Shi et al.16, 17 utilizing a tail-clip injury model comparing the efficacy of platelet-FVIII from 2bF8tg mice and plasma-FVIII in the presence of inhibitors and those of Gewirtz and coworkers.21 Studies by Shi et al.16, 17 demonstrated that platelet-FVIII was markedly more effective than equivalent levels of infused FVIII when inhibitors were infused into the circulation prior to FVIII to mimic the clinical situation of FVIII protein replacement therapy in an inhibitor patient. Further studies showed that the different outcomes were driven by the order of infusions of rhF8 and inhibitors.22 Infusion of rhF8 into FVIIInull mice first followed by antibody infusion allows preassociation of infused FVIII with endogenous VWF in the plasma into a protective VWF/FVIII complex before encountering the inhibitors. In addition, as discussed above, having the SQ sequence containing the furin site in the IbF8 model19, 23, 24 may negatively affect the biological functions of platelet-FVIII. Although preinfusing rhFVIII into FVIIInull mice followed by antibody infusion does not really mimic the treatment of an inhibitor patient, data from Gewirtz and co-workers’ studies21 indicate that the preformed VWF/FVIII complex in platelets (intracellular) is more effective than the complex in plasma (extracellular) when inhibitors are encountered, suggesting that the properties of both platelet storage and VWF/FVIII association are important in platelet gene therapy of hemophilia A with inhibitors.
Another study by Neyman et al.25 using the IbF8 transgenic model, line h38/F8null, showed that the clots formed in IbF8 transgenic mice were less stable than those generated in FVIIInull mice receiving full-length rhFVIII infusion in a laser-induced cremaster vessel injury model. Their subsequent studies demonstrated that functional hemostasis was improved when the residual furin cleavage site within the B-domain was modified.26 Greene et al.27 from the same group developed additional transgenic lines in which FVIII variants including IR8 (inactivation-resistant FVIII) and cB-F8 (canine B-domainless FVIII) were expressed under control of the same GPIbα promoter. The level of platelet-FVIII in cB−F8 transgenic mice was low, only 30% of the hB−F8 or IR8, but it significantly decreased clot embolization in the cremaster injury model.
The PF4 Promoter-Driven Model
Damon et al.28 generated a transgenic mouse model in which a rat PF4 promoter was used to direct human B-domain deleted FVIII expression. FVIII expression was confined to platelets and functional FVIII activity was detected by chromogenic assay in sonicated platelet lysates from transgenic mice with a level of 122 mU/109 platelets. Their studies demonstrated that FVIII stored in platelets is in an inactive form that requires thrombin cleavage for activation. Ectopic expression and storage of FVIII in platelets does not affect the phosphatidylserine exposure or the α-granule release of endogenous platelet-stored proteins. Their studies showed that infusion of thrombopoietin into transgenic mice resulted in a reduction of the amount of FVIII per platelet and clinical efficacy in hemostasis was completely abolished, although the total amount of circulating platelet-FVIII was not affected because platelet number increased. It is unclear why thrombopoietin-induced thrombocytopoiesis diminished the clinical efficacy of platelet-derived FVIII in this transgenic model.
These studies using the transgenic approach provide proof-of-principle that platelet-specific expression of FVIII could be successful for treating hemophilia A even in the presence of inhibitors.
Lentivirus-Mediated Platelet-Specific Gene Therapy of Hemophilia A
To develop a clinically translatable protocol using platelets as a target for gene therapy of hemophilia A, efficient gene transfer and stable expression of FVIII are critical. Since HSCs are the preferable target for gene transfer and lentivirus can efficiently transduce stem cells, utilizing lentiviral vectors to introduce a FVIII expression cassette driven by a platelet lineage-specific promoter should make it possible to establish long-term FVIII expression specifically in megakaryocytes/platelets.
Non-inhibitor Model Studies
Shi et al.29 used a lentivirus-mediated gene transfer system to deliver the 2bF8 expression cassette into HSCs, resulting in FVIII expression in platelets. These studies demonstrated that 2bF8 lentivirus can efficiently introduce functional FVIII expression in megakaryocytic cells. Syngeneic transplantation of 2bF8 lentivirus-transduced bone marrow mononuclear cells from FVIIInull mice into littermates preconditioned with a lethal dose of 11 Gy total-body irradiation (TBI) resulted in sustained therapeutic levels of platelet-FVIII and restored hemostasis with neither inhibitory nor non-inhibitory antibody development through the entire study course. The expression levels of platelet-FVIII in transduced recipients (0.60 ± 0.26 mU/108 platelets) were similar to those obtained in 2bF8tg mice, which were generated by ESC-mediated transgenesis.16 Serial transplants demonstrated that platelet FVIII levels were maintained after sequential bone marrow transplantation, confirming that long-term repopulating HSCs were successfully transduced. These studies build the foundation for lentivirus-mediated platelet-specific gene therapy for hemophilia A.
Greene et al. developed lentiviral vectors harboring hB−F8, IR8, or cB−F8 under control of the GPIbα promoter and introduced FVIII expression by bone marrow transduction and transplantation into FVIIInull mice preconditioned with 10 Gy total-body irradiation.27 They found that the level of platelet-FVIII expression in transduced recipients was about 50% of the level in transgenic mice with both the hB−F8 and IR8 cassettes, but similar low levels were observed in both transgenic and transduced mice with the cB−F8 cassette. Hemostasis was improved in transduced recipients, with better outcomes using IR8 and cBF8 than with hBF8, although protein expression levels were low. Further studies from the same group showed that the low level of FVIII expression in cBF8-transduced recipients was associated with greater megakaryocyte apoptosis than in hBF8-transduced animals. However, when the furin cleavage site in hB−F8 was modified by substituting His for Arg of 1645, the hemostatic efficacy of platelet-derived hB−F8 was significantly improved in the cremaster laser injury model,26 confirming that the residual firin site in their original hB−F8 construct is deleterious to the biological properties of the platelet-FVIII in their IbF8 model.
Inhibitor Model Studies
To investigate whether lentiviral gene delivery to HSCs can introduce therapeutic levels of platelet-FVIII in hemophilia A mice with pre-existing anti-FVIII immunity, Kuether et al.30 transduced enriched HSCs (Sca-1+) from rhFVIII-immunized FVIIInull donor mice with 2bF8 lentivirus followed by transplantation into rhFVIII-immunized FVIIInull recipients. They found that 2bF8 lentivirus-transduced HSCs are viable in recipients with pre-existing anti-FVIII immunity. After transplantation and bone marrow reconstitution, sustained therapeutic levels of platelet-FVIII expression were obtained, while inhibitor titers declined with time in 2bF8 lentivirus-transduced recipients. Hemostatic improvement in the treated animals was confirmed by the tail-clip survival test and the electrolytic induced vessel injury model. The level of platelet-FVIII expression in the inhibitor model was not significantly different from the non-inhibitor model (1.56 ± 0.56 mU/108 platelets versus 1.46 ± 0.43 mU/108 platelets) when a myeloablative conditioning regimen of 11 Gy total body irradiation was employed. There was a wide range of FVIII expression levels among recipients, ranging from 0.36 mU/108 platelets to 6.18 mU/108 platelets. When LAM-PCR (linear amplification-mediated PCR) was used to survey the proviral DNA integration sites in 2bF8-transduced animals, 39 genomic insertion sites were identified in six primary recipients. No over-represented insertion sites were noted, although one (Mds1) corresponds to a common integration site for γ-retroviral vectors31 and another (Arid1a) is a proto-oncogene presented in the Mouse Retrovirus-Tagged Cancer Gene Database.
In this study, Kuether and co-workers30 also evaluated the efficacy of non-myeloablative conditioning regimens, including sublethal total-body irradiation and busulfan chemotherapy, in platelet gene therapy. The levels of platelet-FVIII in the group conditioned with a non-myeloablative total-body irradiation regimen (6.6 Gy) were not significantly different from the group conditioned with myeloablative total-body irradiation (11 Gy). Busulfan, an alkylating agent with potent effects on primitive hematopoietic cells, used as a conditioning alone, resulted in therapeutic levels of platelet-FVIII expression in a 2bF8-transduced non-inhibitor model. However, in the inhibitor model, additional immune suppression, e.g., anti-thymocyte globulin or a low dose of total-body irradiation was required for achieving sufficient 2bF8-genetically manipulated engraftment. These studies demonstrated that 2bF8 lentiviral gene delivery to HSCs can introduce sufficient therapeutic levels of platelet-FVIII expression in hemophilia A mice even with pre-existing anti-FVIII immunity when a sufficient conditioning regimen was employed. These studies suggest that platelet gene therapy may be a promising strategy for gene therapy of hemophilia A even in the high-risk setting of pre-existing anti-FVIII immunity.
Dog Model Studies
To further evaluate the efficacy of platelet gene therapy of hemophilia A, Du et al.32 applied a 2bF8 gene therapy protocol to hemophilia A dogs. Autologous HSCs (CD34+) were enriched from G-CSF (granulocyte colony stimulation factor) and SCF (stem cell factor)-mobilized peripheral blood of hemophilia A dogs, transduced with 2bF8 lentivirus (renamed as 889ITGA2B-BDDFVIII-WPTS in this dog model study) ex vivo, and transplanted back into animals preconditioned with busulfan. Cyclosporine and mycophenolate mofetil were administered for about 90 days after transplantation to prevent immune responses. FVIII was detected in 2bF8 lentivirus-transduced dog platelets and was stored in α-granules although canine platelets do not contain VWF.33, 34 In this study, Du and colleagues32 also examined whether adding a truncated VWF propeptide, which is known to have the capacity to reroute unrelated secreted protein to a storage pathway,35 into the construct (−673ITGA2B-VWFSPD2-BDDFVIII-WPTS) would affect the expression and storage of FVIII in canine platelets. It appeared that FVIII was stored better in α-granules of platelets in an animal that received −673ITGA2B-VWFSPD2-BDDFVIII-WPTS lentivirus-transduced HSCs as determined by electron microscopy, but the platelet-FVIII expression level was similar to that obtained in animals receiving regular 2bF8 lentivirus-transduced HSCs. The levels of platelet-FVIII expression ranged from 5–9 mU/108 platelets. The occurrence of severe bleeding episodes was prevented in all three dogs for at least 2.5 years after transplantation of 2bF8-transduced autologous HSCs. Similar to results in the unimmunized hemophilia A mouse model,29 no anti-FVIII antibodies were detected in dogs that received 2bF8-transduced HSCs. These data demonstrate that 2bF8 lentivirus-mediated HSC transduction followed by autologous transplantation can introduce sustained therapeutic levels of platelet-FVIII resulting in a hemostatic improvement in hemophilia A dogs. Whether the vector containing VWF propeptide would be beneficial for gene therapy of hemophilia A in other species including humans, in which platelets already contain endogenous VWF, will need to be further evaluated.
Human Cell Studies
To evaluate the feasibility of 2bF8 lentivirus-mediated gene delivery to human HSCs for platelet gene therapy of hemophilia A, Shi et al.36 developed an immunocompromised hemophilia A recipient mouse model (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ FVIIInull [NSGF8KO]) and human cord blood (hCB)-derived HSCs were used as a target for 2bF8 gene transfer. CD34+ hCB-derived HSCs were transduced with 2bF8 lentivirus ex vivo and xenotransplanted into NSGF8KO mice preconditioned with busulfan. After xenotransplantation and bone marrow reconstitution, platelet-FVIII was detected in all recipients for as long as human platelet chimerism persisted. Intracellular location of neo-protein FVIII was determined by electron microscopy, demonstrating that FVIII was colocalized with endogenous human VWF in the α-granules of 2bF8-transduced human platelets. When calculated according to chimerism levels measured by flow cytometry, platelet-FVIII level in 2bF8-transduced human platelets (17.77 mU/108 platelets) was significantly greater than those obtained from transduced mouse platelets (1.56 mU/108 platelets).30 The potential reasons leading to this intriguing result include the differences in the size of platelets in humans versus in mice, in the transduction efficiency of 2bF8 lentivirus in human HSCs and mouse counterparts, and in the association properties of human VWF in human platelets versus mouse VWF in murine platelets on stored neo-protein human FVIII. The hemophilic bleeding phenotype was completely rescued in HA mice that received 2bF8 lentivirus-transduced hCB cells in the tail-clip survival test when animals had greater than 2% of platelets derived from 2bF8 lentivirus-transduced hCB cells. Whole blood clotting time analysis confirmed that hemostasis was improved in NSGF8KO mice that received 2bF8LV-transduced hCB cells. When non-restrictive LAM-PCR was used to analyze 2bF8LV insertion sites, no identified integration sites were located within known proto-oncogenes in human hematopoiesis. These studies demonstrate the feasibility of 2bF8 lentiviral gene delivery to hHSCs to introduce FVIII expression in human platelets and that human platelet-FVIII can improve hemostasis in hemophilia A mice.
In Vivo Selection to Enhance Platelet-FVIII Expression
With a goal of improving platelet-FVIII expression and reducing the potential risks associated with platelet gene therapy, such as insertional mutagenesis and preconditioning-related toxicities, Schroeder et al.37 developed a vector, 2bF8/MGMT, which harbors dual genes, the 2bF8 gene and a drug-resistance gene, the P140K O6-methylguanine-DNA-methyltransferase (MGMT P140K) cassette. With this vector, platelet-FVIII expression can be induced by transduction of HSCs at a low MOI followed by transplantation into FVIIInull mice preconditioned with a non-myeloablative regimen. After transplantation, the transduced cells can be enriched in vivo by treatment with O6-benzylguanine (BG) followed by 1,3-bis-2 chloroethyl-1-nitrosourea (BCNU), which selectively kills untransduced hematopoietic cells, and thereby transduced cells are enriched and platelet-FVIII expression is enhanced. Even using a low MOI of 1 and sub-lethal 6.6 Gy total-body irradiation, the level of platelet-FVIII expression in recipients after in vivo selection reached 4.3 ± 5.5 mU/108 platelets, which is 2.9-fold higher than that obtained from nonselectable 2bF8 lentivirus using a typical MOI of 10 with lethal 11Gy total-body irradiation.30 When a typical MOI of 10 was used for transduction, the level of platelet-FVIII expression in BG/BCNU-treated 2bF8/MGMT lentivirus-transduced recipients reached 14.2 ± 12.1 mU/108 platelets. The highest platelet-FVIII expression was 35 mU/108 platelets, which corresponds to 70% of FVIII:C in whole blood in normal mice. Phenotypic correction of the FVIIInull coagulation defect in treated animals was confirmed by a tail bleeding test and rotational thromboelastometry (ROTEM) analysis of the whole blood clotting time.
Importantly, their studies showed that there were no inhibitory or total anti-FVIII immunoglobulin G (IgG) antibodies detected in the BG/BCNU-treated transduced recipients even after exogenous rhFVIII challenge. They found that one of three 2bF8/MGMT-transduced with no BG/BCNU treatment recipients developed a low-titer (6.8 BU/mL) of inhibitors. The one that developed inhibitors was the one with the lowest level of platelet-FVIII expression (0.07 mU/108 platelets, a barely detectable level by chromogenic assay on platelet lysate). These data indicate that achieving a certain level of platelet-FVIII expression is required to induce immune tolerance in transduced recipients. To confirm the immune system was functioning normally in treated animals, recipients were immunized with an unrelated antigen, ovalbumin (OVA). All animals produced high titers of anti-ovalbumin antibodies after ovalbumin immunization, confirming that the immune tolerance developed in BG/BCNU-treated 2bF8/MGMT lentivirus-transduced recipients was FVIII-specific. These data demonstrated that using the MGMT-mediated drug-selection system in 2bF8 gene therapy can efficiently enhance therapeutic platelet-FVIII expression, resulting in sustained phenotypic correction and FVIII-specific immune tolerance in hemophilia A mice even with a low MOI and a non-myeloablative conditioning regimen.
Inducing Platelet-FVIII Expression via In Situ Transduction of HSCs
To avoid preconditioning, Wang and co-workers38 developed a platelet gene therapy protocol utilizing intraosseous delivery of a lentiviral vector that harbors the hFVIII/N6 (human FVIII with a partial B-domain, an N-terminal 226 amino acid stretch [N6]) cassette under control of the human GPIbα promoter (G-F8-LV) or the ubiquitous human elongation factor 1α (EF-1α) promoter (E-F8-LV), to transduce bone marrow cells in situ. Lentiviral vectors were directly injected into FVIIInull mouse tibia through the joint. The studies showed that 3%–20% of FVIII activity was initially detected in plasma by a modified activated thromboplastin time (aPTT) assay in the animals receiving the intraosseous infusion of E-F8-LV, but activity dropped to undetectable levels within 2–3 months due to an anti-FVIII immune response. In contrast, platelets containing FVIII were detectable in animals that received the intraosseous infusion of G-F8-LV up to 160 days. The FVIII antigen level was about 1 mU/108 platelets in the non-inhibitor model and 0.74 mU/108 platelets in the inhibitor model when 2.2 × 107 ifu/animal of G-F8-LV were infused. These studies demonstrate that platelet-FVIII expression can be achieved through in situ transduction of bone marrow cells when FVIII expression is restricted to the platelet lineage.
Collectively, these studies demonstrate that induction of therapeutic levels of platelet-FVIII expression can be successfully achieved using lentivirus-mediated platelet-specific gene delivery to HSCs in hemophilia A even with pre-existing anti-FVIII immunity.
Platelet-Specific Gene Therapy of Hemophilia B
Hemophilia B is an X chromosome-linked recessive bleeding disorder, which results from a factor IX (FIX) deficiency. FIX is a vitamin K-dependent protein that is normally synthesized by hepatocytes. Protein replacement therapy is effective, but it has similar problems to that when used to treat hemophilia A. Although the incidence of anti-FIX inhibitory antibody development is lower in hemophilia B patients after protein replacement therapy, allergic reactions are very common in patients with inhibitors, limiting the use of protein infusion and increasing the risk of morbidity and mortality.39, 40, 41, 42 Gene therapy is a promising approach for hemophilia B. Current clinical trials using adeno-associated virus-mediated liver-restricted FIX expression are very encouraging.43, 44, 45, 46 Phase 1/2 clinical trials showed that dose-dependent expression levels of FIX in circulation were achieved in severe hemophilia B patients after single dose vector administration and the bleeding diathesis was significantly improved. However, for individuals with severe liver disease or neutralizing antibodies to AAV, which are present in 30%–50% of the population,47, 48 an alternative gene therapy approach might be desirable.
Studies from platelet-specific FVIII expression have shown that neo-protein FVIII is stored in platelet α-granules even in the absence of VWF. Thus, a platelet-targeted FIX expression approach was conducted to evaluate the potential of platelet gene therapy of hemophilia B. Zhang and co-workers49 constructed a lentiviral vector (2bF9) in which a hFIX expression cassette is driven by the αIIb promoter, and a transgenic model with platelet-specific FIX expression was generated by 2bF9 lentivirus-mediated transgenesis. Studies using this transgenic model demonstrated that 90% of FIX was stored in platelets and that platelet-derived FIX can rescue the bleeding phenotype in hemophilia B mice in the absence of anti-FIX inhibitors. Platelet-FIX was completely carboxylated and had functional activity, indicating that megakaryocytes/platelets have the capacity to carboxylate the newly synthesized FIX protein precursor to functional FIX protein. The hemostatic efficacy can be transferred by platelet transfusion or bone marrow transplantation. However, unlike platelet-derived FVIII, the clinical efficacy of platelet-FIX is completely abolished when anti-FIX inhibitors are present. This may be due to the lack of a carrier protein to protect FIX as VWF protects FVIII. This would be similar to 2bF8 on the VWF and FVIII double knockout background. Indeed, without VWF, the clinical efficacy of platelet-FVIII is limited in the presence of anti-FVIII inhibitors.18
Although platelet-derived FIX does not maintain clinical efficacy in the presence of anti-FIX inhibitors, targeting FIX expression to platelets could still be a new strategy for gene therapy of hemophilia B. Chen and co-workers50 used 2bF9 lentiviral gene delivery to HSCs by transduction followed by syngeneic transplantation to introduce platelet-FIX expression in hemophilia B mice. Sustained therapeutic levels of platelet-FIX expression were obtained in all recipients that received 2bF9 lentivirus-transduced HSCs. Flow cytometry analysis demonstrated that 6%–39% of platelets expressed FIX in transduced recipients, which was sufficient to restore hemostasis in FIXnull mice in two tail-clipping models, the tail-clip survival test and a tail-bleeding test. Sequential bone marrow transplantation showed that platelet-FIX expression in secondary recipients was sustained, resulting in phenotypic correction. Importantly, none of the 2bF9-transduced recipients developed anti-FIX antibodies after platelet-FIX gene therapy. Only one of nine recipients developed a low titer of inhibitors after challenge with recombinant human FIX in the presence of IFA (incomplete freund adjuvant). These data demonstrate that targeting FIX expression to platelets can restore hemostasis and induce immune tolerance in hemophilia B mice, suggesting that platelet gene therapy may be a promising strategy for gene therapy of hemophilia B in humans.
The Safety Issues and Perspective in Platelet Gene Therapy
Several safety issues should be considered while conducting any platelet-specific gene therapy protocol for hemophilia A. Since FVIII is not normally expressed in platelets and platelets play fundamental roles in both hemostasis and thrombosis, it is important to evaluate whether ectopic expression of FVIII in platelets would have a potential thrombotic risk. To evaluate this critical safety issue, several new lines of 2bF8 transgenic mice (LV17tg, LV18tg, and LV17/18tg)51, 52 were generated using 2bF8 lentivirus-mediated transgenesis in an effort to establish a line with a high level of platelet-FVIII. Baumgartner and colleagues51 used a line, LV17/18tg, that expresses 30-fold higher platelet-FVIII levels than therapeutically required to restore hemostasis in hemophilic mice to assess the hemostatic properties of platelet-FVIII with a variety of techniques, including native whole-blood thrombin generation, ex vivo and in vivo clot formation, assessment of plasma parameters associated with increased thrombosis risk (D-dimer, thrombin anti-thrombin complexes, fibrinogen), tissue fibrin deposition, platelet activation status and activatability, and evaluation of platelet-leukocyte aggregates. Their data demonstrated that in steady state as well as under prothrombotic conditions induced by lipopolysaccharides (LPS)-mediated inflammation or the factor V Leiden mutation, supratherapeutic levels of platelet-FVIII appeared nonthrombogenic. Furthermore, FVIII-expressing platelets were neither hyperactivated nor hyperactivatable upon agonist activation. Their studies demonstrated that no thrombotic risk was identified with supratherapeutic levels of platelet-FVIII in mouse models. Thus, in this regard, platelet-targeted FVIII gene therapy can be considered a safe therapy with a relatively wide therapeutic window.
One concern for gene therapy is the potential for an immune response to the transgene product or viral proteins. Immune responses may result in gene therapy failure because the transfected cells may be eliminated if cellular responses occur, or the functional bioactivity of the neo-protein may be inactivated if a humoral response is induced. It has been reported that immune responses were a problem in lentivirus-mediated HSC gene therapy when FVIII transgene expression is directed by ubiquitous promoters.38, 53 This problem is not encountered when FVIII expression is targeted to and stored in platelets under control of the αIIb promoter.29, 30, 37, 54 Instead, lentivirus-mediated αIIb promoter directed platelet-specific gene therapy can efficiently promote antigen-specific immune tolerance in both hemophilia A and B mouse models.37, 50, 54 Studies by Chen and colleagues54 show that CD4 T cell compartment is tolerized after 2bF8 gene therapy. They found that T regulatory (Treg) cells significantly increased in 2bF8-transduced recipients compared to untransduced animals and that the immune tolerance was transferable through adoptive transfer of splenocytes. These data indicate FVIII-specific Treg cells are induced in animals after platelet-specific gene therapy, which could be a mechanism of the immune tolerance established after platelet gene therapy. To investigate the immunogenicity of platelet-FVIII, Chen and colleagues52 transfused 2bF8tg platelets into naive or primed FVIIInull mice and monitored anti-FVIII immune responses. They found that platelet containing FVIII triggers neither primary nor secondary immune responses, indicating that platelet-FVIII is well insulated from exposure to the immune system, avoiding activation of the immune response.
Another concern is genotoxicity resulting from viral vector insertion site-related mutagenesis. Potential genotoxicity is associated with gene therapy when an integrating viral vector is used to introduce transgene expression. The advanced self-inactivating design of lentiviral vectors and the differences in integration site selection between lentiviral and oncoretroviral vectors may reduce the risk of insertional mutagenesis. Oncoretroviral vectors tend to integrate preferentially near promoter-proximal regions, with hotspots in proto-oncogenes or genes related to cell proliferation. In contrast, lentiviral vectors appear to integrate more randomly into open chromatin without hotspots.55, 56 While the studies utilizing LAM-PCR to survey 2bF8 integrants showed that there are no over-represented insertion sites in animals that received 2bF8-transduced HSCs from mice,30 dogs,32 or humans,36 onco-mutagenesis resulting from the random integration of the transgene into the genome remains a potential risk in lentivirus-mediated gene transfer. Further studies using high-throughput techniques to more thoroughly determine the integration repertoire of these vectors are required.
In addition, occurrence of abnormal apoptosis in megakaryocytes when FVIII is ectopically expressed in the platelet lineage has been reported.26 Studies done by Greene and co-workers26 showed that platelet-targeted B-domainless FVIII expression under control of the GPIbα gene promoter increases apoptosis in transduced megakaryocytes, resulting in a 30% reduction of platelet counts in transduced recipients. This does not seem to be the case in the 2bF8 model, in which the complete B-domain-deleted FVIII cassette without the SQ sequence is used and FVIII expression is driven by the αIIb promoter. Studies by Schroeder et al.37 using the 2bF8/MGMT system have demonstrated that platelet number in 2bF8/MGMT lentivirus-transduced animals had fully recovered within 8 weeks after transplantation when a non-myeloablative conditioning regimen was applied, and platelet counts were normal in transduced animals even with FVIII expression as high as 14.18 ± 12.05 mU per 108 platelets after in vivo drug selection, which corresponds to about 23% of FVIII activity in normal mouse whole blood. It is unclear whether the cellular toxicity of FVIII expression in megakaryocytes in the IbF8 model is a specific phenotype associated with the residual SQ sequence that includes the furin cleavage site from the B-domain or is a phenotype driven by the GPIbα promoter. Further studies are needed.
In summary, studies from preclinical trials using animal models have demonstrated the feasibility of platelet delivery of therapeutics for disease treatment through genetic manipulation of HSCs. Data from the human clinical trial phase I/II using liver-specific AAV-mediated codon-optimized FVIII (AAV5-hFVIII-SQ) gene therapy are very encouraging.57 However, patients who have severe liver diseases, neutralizing antibodies to AAV, or a history of FVIII inhibitor development are excluded from the AAV-mediated liver-targeted gene therapy protocol. Targeting FVIII or FIX expression and storage to platelets is a promising strategy for gene therapy of hemophilia, with the potential to not only improve hemostasis but also induce immune tolerance. In a clinical scenario, patient-derived HSCs will be harvested from mobilized peripheral blood or cord blood for ex vivo manipulation to introduce a corrected FVIII or FIX gene driven by a platelet-specific promoter, followed by autologous transplantation. This is a promising approach for gene therapy of hemophilia A patients with inhibitors as well as non-hemophilic patients with acquired inhibitory antibodies who can also have life-threatening clinical bleeding which is difficult to treat by conventional therapies.
Acknowledgments
The author acknowledges support from the National Heart Lung and Blood Institute of the NIH grant HL102035, American Heart Association grants (0320043Z, 0520035Z, and SDG 0730183N), National Hemophilia Foundation Career Development Award, a Hemophilia Association of New York grant, and kind gifts from Midwest Athletes Against Childhood Cancer and Bleeding Disorder Fund and Children’s Hospital of Wisconsin Research Foundation.
References
- 1.Chavin S.I. Factor VIII: structure and function in blood clotting. Am. J. Hematol. 1984;16:297–306. doi: 10.1002/ajh.2830160312. [DOI] [PubMed] [Google Scholar]
- 2.Wong T., Recht M. Current options and new developments in the treatment of haemophilia. Drugs. 2011;71:305–320. doi: 10.2165/11585340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Mannucci P.M., Mancuso M.E., Santagostino E. How we choose factor VIII to treat hemophilia. Blood. 2012;119:4108–4114. doi: 10.1182/blood-2012-01-394411. [DOI] [PubMed] [Google Scholar]
- 4.Iorio A., Halimeh S., Holzhauer S., Goldenberg N., Marchesini E., Marcucci M., Young G., Bidlingmaier C., Brandao L.R., Ettingshausen C.E. Rate of inhibitor development in previously untreated hemophilia A patients treated with plasma-derived or recombinant factor VIII concentrates: a systematic review. J. Thromb. Haemost. 2010;8:1256–1265. doi: 10.1111/j.1538-7836.2010.03823.x. [DOI] [PubMed] [Google Scholar]
- 5.Mancuso M.E., Mannucci P.M., Rocino A., Garagiola I., Tagliaferri A., Santagostino E. Source and purity of factor VIII products as risk factors for inhibitor development in patients with hemophilia A. J. Thromb. Haemost. 2012;10:781–790. doi: 10.1111/j.1538-7836.2012.04691.x. [DOI] [PubMed] [Google Scholar]
- 6.Franchini M., Coppola A., Rocino A., Santagostino E., Tagliaferri A., Zanon E., Morfini M., Italian Association of Hemophilia Centers (AICE) Working Group Systematic review of the role of FVIII concentrates in inhibitor development in previously untreated patients with severe hemophilia a: a 2013 update. Semin. Thromb. Hemost. 2013;39:752–766. doi: 10.1055/s-0033-1356715. [DOI] [PubMed] [Google Scholar]
- 7.White G.C., 2nd, McMillan C.W., Blatt P.M., Roberts H.R. Factor VIII inhibitors: a clinical overview. Am. J. Hematol. 1982;13:335–342. doi: 10.1002/ajh.2830130410. [DOI] [PubMed] [Google Scholar]
- 8.Scandella D.H. Properties of anti-factor VIII inhibitor antibodies in hemophilia A patients. Semin. Thromb. Hemost. 2000;26:137–142. doi: 10.1055/s-2000-9815. [DOI] [PubMed] [Google Scholar]
- 9.Furie B., Limentani S.A., Rosenfield C.G. A practical guide to the evaluation and treatment of hemophilia. Blood. 1994;84:3–9. [PubMed] [Google Scholar]
- 10.Shi Q., Wilcox D.A., Fahs S.A., Kroner P.A., Montgomery R.R. Expression of human factor VIII under control of the platelet-specific alphaIIb promoter in megakaryocytic cell line as well as storage together with VWF. Mol. Genet. Metab. 2003;79:25–33. doi: 10.1016/s1096-7192(03)00049-0. [DOI] [PubMed] [Google Scholar]
- 11.Shi Q., Fahs S.A., Kuether E.L., Cooley B.C., Weiler H., Montgomery R.R. Targeting FVIII expression to endothelial cells regenerates a releasable pool of FVIII and restores hemostasis in a mouse model of hemophilia A. Blood. 2010;116:3049–3057. doi: 10.1182/blood-2010-03-272419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lind P., Larsson K., Spira J., Sydow-Bäckman M., Almstedt A., Gray E., Sandberg H. Novel forms of B-domain-deleted recombinant factor VIII molecules. Construction and biochemical characterization. Eur. J. Biochem. 1995;232:19–27. doi: 10.1111/j.1432-1033.1995.tb20776.x. [DOI] [PubMed] [Google Scholar]
- 13.Siner J.I., Iacobelli N.P., Sabatino D.E., Ivanciu L., Zhou S., Poncz M., Camire R.M., Arruda V.R. Minimal modification in the factor VIII B-domain sequence ameliorates the murine hemophilia A phenotype. Blood. 2013;121:4396–4403. doi: 10.1182/blood-2012-10-464164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siner J.I., Samelson-Jones B.J., Crudele J.M., French R.A., Lee B.J., Zhou S., Merricks E., Raymer R., Nichols T.C., Camire R.M., Arruda V.R. Circumventing furin enhances factor VIII biological activity and ameliorates bleeding phenotypes in hemophilia models. JCI Insight. 2016;1:e89371. doi: 10.1172/jci.insight.89371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen G.N., George L.A., Siner J.I., Davidson R.J., Zander C.B., Zheng X.L., Arruda V.R., Camire R.M., Sabatino D.E. Novel factor VIII variants with a modified furin cleavage site improve the efficacy of gene therapy for hemophilia A. J. Thromb. Haemost. 2017;15:110–121. doi: 10.1111/jth.13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Q., Wilcox D.A., Fahs S.A., Weiler H., Wells C.W., Cooley B.C., Desai D., Morateck P.A., Gorski J., Montgomery R.R. Factor VIII ectopically targeted to platelets is therapeutic in hemophilia A with high-titer inhibitory antibodies. J. Clin. Invest. 2006;116:1974–1982. doi: 10.1172/JCI28416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Q., Fahs S.A., Wilcox D.A., Kuether E.L., Morateck P.A., Mareno N., Weiler H., Montgomery R.R. Syngeneic transplantation of hematopoietic stem cells that are genetically modified to express factor VIII in platelets restores hemostasis to hemophilia A mice with preexisting FVIII immunity. Blood. 2008;112:2713–2721. doi: 10.1182/blood-2008-02-138214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Q., Schroeder J.A., Kuether E.L., Montgomery R.R. The important role of von Willebrand factor in platelet-derived FVIII gene therapy for murine hemophilia A in the presence of inhibitory antibodies. J. Thromb. Haemost. 2015;13:1301–1309. doi: 10.1111/jth.13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yarovoi H.V., Kufrin D., Eslin D.E., Thornton M.A., Haberichter S.L., Shi Q., Zhu H., Camire R., Fakharzadeh S.S., Kowalska M.A. Factor VIII ectopically expressed in platelets: efficacy in hemophilia A treatment. Blood. 2003;102:4006–4013. doi: 10.1182/blood-2003-05-1519. [DOI] [PubMed] [Google Scholar]
- 20.Yarovoi H., Nurden A.T., Montgomery R.R., Nurden P., Poncz M. Intracellular interaction of von Willebrand factor and factor VIII depends on cellular context: lessons from platelet-expressed factor VIII. Blood. 2005;105:4674–4676. doi: 10.1182/blood-2004-12-4701. [DOI] [PubMed] [Google Scholar]
- 21.Gewirtz J., Thornton M.A., Rauova L., Poncz M. Platelet-delivered factor VIII provides limited resistance to anti-factor VIII inhibitors. J. Thromb. Haemost. 2008;6:1160–1166. doi: 10.1111/j.1538-7836.2008.02992.x. [DOI] [PubMed] [Google Scholar]
- 22.Shi Q., Kuether E.L., Schroeder J.A., Perry C.L., Fahs S.A., Cox Gill J., Montgomery R.R. Factor VIII inhibitors: von Willebrand factor makes a difference in vitro and in vivo. J. Thromb. Haemost. 2012;10:2328–2337. doi: 10.1111/j.1538-7836.2012.04902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fakharzadeh S.S., Zhang Y., Sarkar R., Kazazian H.H., Jr. Correction of the coagulation defect in hemophilia A mice through factor VIII expression in skin. Blood. 2000;95:2799–2805. [PubMed] [Google Scholar]
- 24.Toole J.J., Pittman D.D., Orr E.C., Murtha P., Wasley L.C., Kaufman R.J. A large region (approximately equal to 95 kDa) of human factor VIII is dispensable for in vitro procoagulant activity. Proc. Natl. Acad. Sci. USA. 1986;83:5939–5942. doi: 10.1073/pnas.83.16.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neyman M., Gewirtz J., Poncz M. Analysis of the spatial and temporal characteristics of platelet-delivered factor VIII-based clots. Blood. 2008;112:1101–1108. doi: 10.1182/blood-2008-04-152959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greene T.K., Lyde R.B., Bailey S.C., Lambert M.P., Zhai L., Sabatino D.E., Camire R.M., Arruda V.R., Poncz M. Apoptotic effects of platelet factor VIII on megakaryopoiesis: implications for a modified human FVIII for platelet-based gene therapy. J. Thromb. Haemost. 2014;12:2102–2112. doi: 10.1111/jth.12749. [DOI] [PubMed] [Google Scholar]
- 27.Greene T.K., Wang C., Hirsch J.D., Zhai L., Gewirtz J., Thornton M.A., Miao H.Z., Pipe S.W., Kaufman R.J., Camire R.M. In vivo efficacy of platelet-delivered, high specific activity factor VIII variants. Blood. 2010;116:6114–6122. doi: 10.1182/blood-2010-06-293308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damon A.L., Scudder L.E., Gnatenko D.V., Sitaraman V., Hearing P., Jesty J., Bahou W.F. Altered bioavailability of platelet-derived factor VIII during thrombocytosis reverses phenotypic efficacy in haemophilic mice. Thromb. Haemost. 2008;100:1111–1122. doi: 10.1160/th08-04-0242. [DOI] [PubMed] [Google Scholar]
- 29.Shi Q., Wilcox D.A., Fahs S.A., Fang J., Johnson B.D., Du L.M., Desai D., Montgomery R.R. Lentivirus-mediated platelet-derived factor VIII gene therapy in murine haemophilia A. J. Thromb. Haemost. 2007;5:352–361. doi: 10.1111/j.1538-7836.2007.02346.x. [DOI] [PubMed] [Google Scholar]
- 30.Kuether E.L., Schroeder J.A., Fahs S.A., Cooley B.C., Chen Y., Montgomery R.R., Wilcox D.A., Shi Q. Lentivirus-mediated platelet gene therapy of murine hemophilia A with pre-existing anti-factor VIII immunity. J. Thromb. Haemost. 2012;10:1570–1580. doi: 10.1111/j.1538-7836.2012.04791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calmels B., Ferguson C., Laukkanen M.O., Adler R., Faulhaber M., Kim H.J., Sellers S., Hematti P., Schmidt M., von Kalle C. Recurrent retroviral vector integration at the Mds1/Evi1 locus in nonhuman primate hematopoietic cells. Blood. 2005;106:2530–2533. doi: 10.1182/blood-2005-03-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du L.M., Nurden P., Nurden A.T., Nichols T.C., Bellinger D.A., Jensen E.S., Haberichter S.L., Merricks E., Raymer R.A., Fang J. Platelet-targeted gene therapy with human factor VIII establishes haemostasis in dogs with haemophilia A. Nat. Commun. 2013;4:2773. doi: 10.1038/ncomms3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nichols T.C., Samama C.M., Bellinger D.A., Roussi J., Reddick R.L., Bonneau M., Read M.S., Bailliart O., Koch G.G., Vaiman M. Function of von Willebrand factor after crossed bone marrow transplantation between normal and von Willebrand disease pigs: effect on arterial thrombosis in chimeras. Proc. Natl. Acad. Sci. USA. 1995;92:2455–2459. doi: 10.1073/pnas.92.7.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanders W.E., Jr., Reddick R.L., Nichols T.C., Brinkhous K.M., Read M.S. Thrombotic thrombocytopenia induced in dogs and pigs. The role of plasma and platelet vWF in animal models of thrombotic thrombocytopenic purpura. Arterioscler. Thromb. Vasc. Biol. 1995;15:793–800. doi: 10.1161/01.atv.15.6.793. [DOI] [PubMed] [Google Scholar]
- 35.Haberichter S.L., Jozwiak M.A., Rosenberg J.B., Christopherson P.A., Montgomery R.R. The von Willebrand factor propeptide (VWFpp) traffics an unrelated protein to storage. Arterioscler. Thromb. Vasc. Biol. 2002;22:921–926. doi: 10.1161/01.atv.0000017063.36768.87. [DOI] [PubMed] [Google Scholar]
- 36.Shi Q., Kuether E.L., Chen Y., Schroeder J.A., Fahs S.A., Montgomery R.R. Platelet gene therapy corrects the hemophilic phenotype in immunocompromised hemophilia A mice transplanted with genetically manipulated human cord blood stem cells. Blood. 2014;123:395–403. doi: 10.1182/blood-2013-08-520478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schroeder J.A., Chen Y., Fang J., Wilcox D.A., Shi Q. In vivo enrichment of genetically manipulated platelets corrects the murine hemophilic phenotype and induces immune tolerance even using a low multiplicity of infection. J. Thromb. Haemost. 2014;12:1283–1293. doi: 10.1111/jth.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X., Shin S.C., Chiang A.F., Khan I., Pan D., Rawlings D.J., Miao C.H. Intraosseous delivery of lentiviral vectors targeting factor VIII expression in platelets corrects murine hemophilia A. Mol. Ther. 2015;23:617–626. doi: 10.1038/mt.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jadhav M., Warrier I. Anaphylaxis in patients with hemophilia. Semin. Thromb. Hemost. 2000;26:205–208. doi: 10.1055/s-2000-9824. [DOI] [PubMed] [Google Scholar]
- 40.Lusher J.M. Inhibitor antibodies to factor VIII and factor IX: management. Semin. Thromb. Hemost. 2000;26:179–188. doi: 10.1055/s-2000-9821. [DOI] [PubMed] [Google Scholar]
- 41.Shibata M., Shima M., Misu H., Okimoto Y., Giddings J.C., Yoshioka A. Management of haemophilia B inhibitor patients with anaphylactic reactions to FIX concentrates. Haemophilia. 2003;9:269–271. doi: 10.1046/j.1365-2516.2003.00772.x. [DOI] [PubMed] [Google Scholar]
- 42.Franchini M., Lippi G., Montagnana M., Targher G., Zaffanello M., Salvagno G.L., Rivolta G.F., Di Perna C., Tagliaferri A. Anaphylaxis in patients with congenital bleeding disorders and inhibitors. Blood Coagul. Fibrinolysis. 2009;20:225–229. doi: 10.1097/MBC.0b013e328329f265. [DOI] [PubMed] [Google Scholar]
- 43.Nathwani A.C., Tuddenham E.G., Rangarajan S., Rosales C., McIntosh J., Linch D.C., Chowdary P., Riddell A., Pie A.J., Harrington C. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nathwani A.C., Reiss U.M., Tuddenham E.G., Rosales C., Chowdary P., McIntosh J., Della Peruta M., Lheriteau E., Patel N., Raj D. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.George L.A., Sullivan S.K., Giermasz A., Rasko J.E.J., Samelson-Jones B.J., Ducore J., Cuker A., Sullivan L.M., Majumdar S., Teitel J. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N. Engl. J. Med. 2017;377:2215–2227. doi: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miesbach W., Meijer K., Coppens M., Kampmann P., Klamroth R., Schutgens R., Tangelder M., Castaman G., Schwäble J., Bonig H. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood. 2017 doi: 10.1182/blood-2017-09-804419. blood-2017-09-804419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calcedo R., Vandenberghe L.H., Gao G., Lin J., Wilson J.M. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calcedo R., Morizono H., Wang L., McCarter R., He J., Jones D., Batshaw M.L., Wilson J.M. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin. Vaccine Immunol. 2011;18:1586–1588. doi: 10.1128/CVI.05107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang G., Shi Q., Fahs S.A., Kuether E.L., Walsh C.E., Montgomery R.R. Factor IX ectopically expressed in platelets can be stored in alpha-granules and corrects the phenotype of hemophilia B mice. Blood. 2010;116:1235–1243. doi: 10.1182/blood-2009-11-255612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y., Schroeder J.A., Kuether E.L., Zhang G., Shi Q. Platelet gene therapy by lentiviral gene delivery to hematopoietic stem cells restores hemostasis and induces humoral immune tolerance in FIX(null) mice. Mol. Ther. 2014;22:169–177. doi: 10.1038/mt.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baumgartner C.K., Mattson J.G., Weiler H., Shi Q., Montgomery R.R. Targeting factor VIII expression to platelets for hemophilia A gene therapy does not induce an apparent thrombotic risk in mice. J. Thromb. Haemost. 2017;15:98–109. doi: 10.1111/jth.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y., Schroeder J.A., Chen J., Luo X., Baumgartner C.K., Montgomery R.R., Hu J., Shi Q. The immunogenicity of platelet-derived FVIII in hemophilia A mice with or without preexisting anti-FVIII immunity. Blood. 2016;127:1346–1354. doi: 10.1182/blood-2015-08-662916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kootstra N.A., Matsumura R., Verma I.M. Efficient production of human FVIII in hemophilic mice using lentiviral vectors. Mol. Ther. 2003;7:623–631. doi: 10.1016/s1525-0016(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y., Luo X., Schroeder J.A., Chen J., Baumgartner C.K., Hu J., Shi Q. Immune tolerance induced by platelet-targeted factor VIII gene therapy in hemophilia A mice is CD4 T cell mediated. J. Thromb. Haemost. 2017;15:1994–2004. doi: 10.1111/jth.13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beard B.C., Dickerson D., Beebe K., Gooch C., Fletcher J., Okbinoglu T., Miller D.G., Jacobs M.A., Kaul R., Kiem H.P., Trobridge G.D. Comparison of HIV-derived lentiviral and MLV-based gammaretroviral vector integration sites in primate repopulating cells. Mol. Ther. 2007;15:1356–1365. doi: 10.1038/sj.mt.6300159. [DOI] [PubMed] [Google Scholar]
- 56.Montini E., Cesana D., Schmidt M., Sanvito F., Ponzoni M., Bartholomae C., Sergi Sergi L., Benedicenti F., Ambrosi A., Di Serio C. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat. Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 57.Rangarajan S., Walsh L., Lester W., Perry D., Madan B., Laffan M., Yu H., Vettermann C., Pierce G.F., Wong W.Y., Pasi K.J. AAV5-factor VIII gene transfer in severe hemophilia A. N. Engl. J. Med. 2017;377:2519–2530. doi: 10.1056/NEJMoa1708483. [DOI] [PubMed] [Google Scholar]