Figure 3.
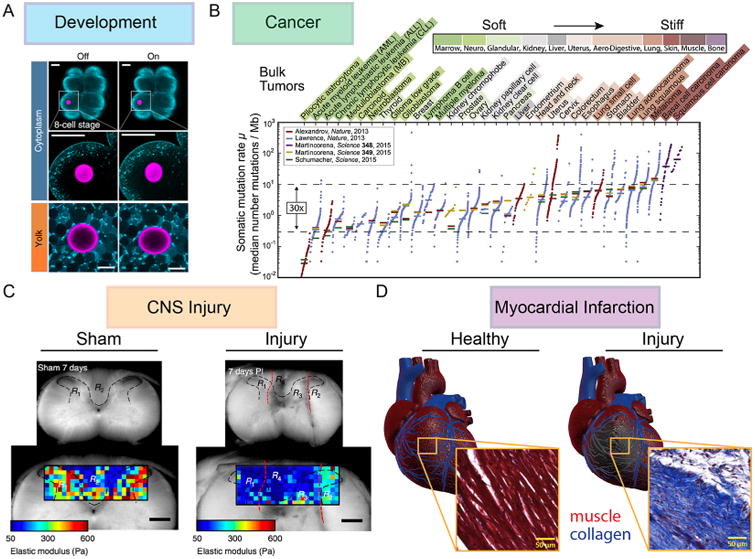
Unique cell–matrix microenvironments. (A) In the developing embryo, stiffness gradients begin to appear as early as the blastula phase. Using ferrofluid microdroplets as mechanical actuators, Serwane et al. showed that droplet deformation under identical magnetic fields yields more deformation in the cytoplasm of a blastomere than in the yolk, indicating a stiffer yolk. These droplets can be actuated dynamically during the entire course of embryo development to measure viscoelastic properties of embryonic tissues.102 (B) Pfeifer et al. recently investigated the cancer cell–ECM microenvironment by finding a correlation between the stiffness of the tissue surrounding a tumor and the somatic mutation rate within the tumor.80 This has been hypothesized to be the result of increased ECM deposition in stiffer tissues requiring migrating cancer cells to contort their nuclei, causing a depletion of DNA repair factor and a subsequent increase in DNA damage.103 (C) Clinical translation of mechanobiology research to the field of CNS regeneration is an urgent need. Atomic force microscopy analysis of both uninjured regions and stab injury sites of the neocortex performed in Moeendarbary et al. revealed that brain tissue softens after injury, and that this softening extends to regions nearly half a millimeter away from the injury and persists for over 3 weeks.104 (D) Another potential clinical application for mechanobiology principles is in myocardial infarction, where cell death in the infarct zone leads to increased matrix deposition and stiffening. This ECM alteration results in decreased cardiac output for post-MI patients.105 Images adapted from refs 80, 102, 104, and 105.
