Fig. 7.
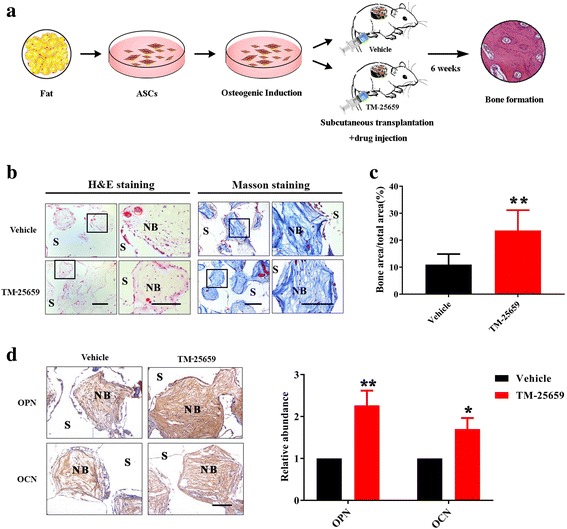
TM-25659 delivery by oral gavage promotes in vivo bone formation of ADSCs. a Schematic description of the experimental procedure for in vivo transplantation. Adipose-derived stem cells (ASCs) were initially treated with osteoinductive medium for 7 consecutive days and then harvested, seeded on porous β-TCP blocks as carriers, and subcutaneously transplanted into nude mice (six animals per experimental group). Six weeks later all transplants were harvested for further analysis. b Hematoxylin and eosin (H&E) and Masson trichrome staining revealed markedly enhanced bone formation in samples from animals treated with TM-25659 via oral gavage compared with vehicle-treated samples. Scale bar = 100 μm. c Quantification of bone formation in samples indicated significantly more bone formation in samples from animals treated with TM-25659. Ten images of Masson staining (400×) were randomly selected in the slides from two experimental groups and captured under microscopy. The area of new bone in each image was marked using ImageJ and the percentage of new bone over total area was calculated. d Immunohistochemical staining of osteopontin (OPN) and osteocalcin (OCN) in samples revealed elevated OPN and OCN abundance in samples from animals treated with TM-25659 compared with vehicle-treated samples. Scale bar = 100 μm. Data are shown as fold-change compared with vehicle-treated samples which were defined as 1.0. Data shown here are mean ± SD from two independent experiments; *P < 0.05, **P < 0.01, by Student’s t test. NB new bone, S scaffold
