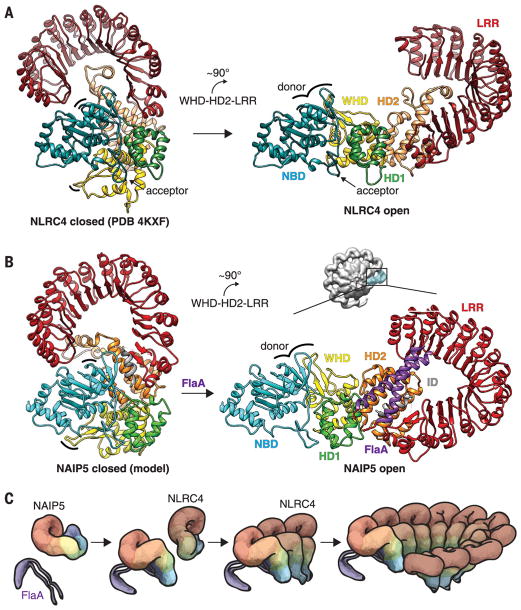
Fig. 3. Model of NAIP5-NLRC4 inflammasome assembly.
(A) Structures of inactive (left) and active (right, determined in this work) NLRC4, showing a ~90° rigid-body rotation of the WHD-HD2-LRR module (9, 10) triggered by interaction with an activated NAIP5 or another already activated NLRC4. (B) To generate the inactive NAIP5 conformation [left; modeled based on NLRC4 (methods)], the HD2 insert was moved to avoid collision with the NBD. We propose that flagellin binding induces a ~90° rigid-body rotation of the WHD-HD2-LRR module, analogous to the rotation of NLRC4, which displaces the occluding LRR and HD2 from the NBD to complete and expose the donor oligomerization surface (indicated by curved lines in left and right panels) for interaction with NLRC4. (C) Proposed events of inflammasome assembly. The flagellin D0 domain (purple) binds to NAIP5 and unfurls the protein for subsequent NLRC4 recruitment and activation. Active NLRC4 recruits further NLRC4 protomers for self-propagating oligomerization and completion of a caspase-1 recruitment platform. Colors are as in Fig. 1A.