Abstract
Objective
To compare the physiological stress responses of infants born <30 weeks’ gestational age when undergoing clustered nursing cares with standardised neurobehavioural assessments in neonatal nurseries.
Design/methods
Thirty-four infants born <30 weeks’ gestation were recruited from a tertiary neonatal intensive care unit. Heart rate (HR) and oxygen saturation were recorded during clustered nursing cares and during standardised neurobehavioural assessments (including the General Movements Assessment, Hammersmith Neonatal Neurological Examination and Premie-Neuro Assessment). Two assessors extracted HR and oxygen saturations at 5 s intervals, with HR instability defined either as tachycardia (HR >180 beats per minute (bpm)) or bradycardia (HR <100 bpm). Oxygen desaturations were defined as SpO2<90%. Physiological stability was compared between nursing cares and neurobehavioural assessments using linear (for continuous outcomes) and logistic (HR instability and oxygen desaturation) regression.
Results
Compared with clustered nursing cares HR was lower (mean difference −5.9 bpm; 95% CI −6.5 to 5.3; P<0.001) and oxygen saturation higher (mean difference 2.4%; 95% CI 2.1% to 2.6%; P<0.001) during standardised neurobehavioural assessments. Compared with clustered nursing cares neurobehavioural assessments were also associated with reduced odds of tachycardia (OR 0.44, 95% CI 0.22 to 0.86), HR instability (OR 0.43, 95% CI 0.22 to 0.85) and oxygen desaturation (OR 0.43, 95% CI 0.26 to 0.70).
Conclusions
Standardised neurobehavioural assessments are associated with less physiological stress than clustered nursing cares in infants aged 29–32 weeks’ postmenstrual age, and are therefore possible without causing undue physiological disturbance in medically stable infants.
Keywords: neonatology, nursing care, occupational therapy, physical therapy, neuro development
What is already known on this topic?
Exposure to stressful procedures/events including handling occurs daily in neonatal nurseries for very preterm infants.
Strong links exist between brain development and cumulative stress, leading to adverse long-term neurodevelopmental outcome.
What this study hopes to add?
Standardised neurobehavioural assessments are associated with less physiological stress than clustered nursing cares.
Standardised neurobehavioural assessments requiring handling caused more instability than standardised neurobehavioural assessments that did not require handling.
Introduction
Very preterm infants (<30 weeks’ gestation) experience many events that are associated with stress to their systems, and which occur during a time of rapid brain growth and organisation.1–3 On average, preterm infants in the neonatal intensive care unit (NICU) are exposed to two invasive procedures a day, with some infants having as many as 10.4 The number and severity of these stressors have been shown to influence cortical connectivity, potentially setting the scene for later abnormal development and learning.2 3 5–8 Stress in this context can be characterised as any threat or perceived threat of a physical or psychological nature to the stability of a very preterm infants' homeostasis.9 Light, noise, handling/touch stimulation, clustered nursing cares (clustering several routine or nursing care events together rather than spacing them out over time), neurobehavioural/neurological assessments and medical procedures can all be considered environmental stressors.2 5 10–13 For the very preterm infant, there is therefore a delicate balance between limiting exposure to these environmental stressors, while at the same time providing essential care.
Standardised neurobehavioural and neurological assessments are increasingly being used throughout the neonatal period in very preterm infants, and together with neuroimaging and other medical indicators, can assist in determining the need for early intervention. The physiological stress associated with neurobehavioural/neurological assessments, however, requires further investigation and in particular a comparison with clustered nursing cares has not been performed.
Therefore, the primary aim of the current study was to compare the physiological stress of very preterm infants undergoing clustered nursing cares versus standardised neurobehavioural assessments to assess whether these assessments inflict additional stress on the infant. A secondary aim was to explore whether physiological stress differed between different neurobehavioural assessments that did or did not require handling.
Methods
This was a single-centre, prospective, within-subject, observational study, reported using the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.14 15 Participants were a subset of infants born <30 weeks' gestation between January 2011 and December 2013, recruited as part of a larger serial neurobehaviour study from the neonatal nurseries at the Royal Women’s Hospital in Melbourne, a tertiary level neonatal intensive and special care unit.16 Infants were excluded if they had congenital abnormalities known to affect neurodevelopment, had non-English-speaking parents, or who were medically unstable. Informed parental consent was obtained for all participants.
The physiological stress imposed by standardised neurobehavioural assessments was compared with that during clustered nursing cares. Three standardised neurobehavioural assessments, the General Movements Assessment (GM),17 the Premie-Neuro Assessment (PN)18 and the Hammersmith Neonatal Neurological Examination (HNNE),19 were administered weekly from birth until 32 weeks’ postmenstrual age.16 The PN and the HNNE were chosen to provide neurological and neurobehavioural data required for the main study. The GMs were chosen to provide further neurological information through observation alone.
Assessments were timed with clustered nursing cares, classified as any task or procedure necessary for the continued ongoing daily care of the infant, performed by a parent, nursing staff or a combination of the two. Neurobehavioural assessments were administered by trained and certified assessors following a standardised procedure,16 commencing with the GMs, which involved only videoing the infant’s spontaneous movement in supine for approximately 5 min, then the PN and the HNNE, as tolerated by the infant. The PN and HNNE both involve handling the infant and share numerous items. To minimise repeated handling and accumulative stress, shared items were administered once during the PN, with only the additional items captured in the HNNE, which was administered last. Clustered nursing cares usually took precedence over the standardised neurobehavioural assessments, as they are an essential component of care. The bedside nurse determined the order based on the infant’s needs and their workload.
All assessments and nursing cares were video recorded using a digital video camera mounted on a portable pole and positioned above the incubator/open cot, avoiding the infant’s direct vision and allowing staff access. A pulse oximeter sensor (LNOP Neo-L, Masimo, Irvine, CA, USA) was placed around the infant’s foot or wrist and connected to an oximeter (Masimo SET, Masimo). The pulse oximeter was set to maximum sensitivity with 2 s averaging to provide rapid detection of changes in oxygen saturation (SpO2), heart rate (HR) and signal quality. The pulse oximeter was positioned inside the incubator/open cot within the camera’s view to monitor HR and SpO2.
Recordings containing both clustered nursing cares and standardised neurobehavioural assessments completed between 29 and 32 weeks’ postmenstrual age were reviewed by the first author (LGA). Videos were excluded if the pulse oximeter was out of camera view, had distorted readings, or was obstructed by anything or anyone (assessors/infants/nursing staff/parents). Video contents were divided into individual nursing cares and individual standardised neurobehavioural assessments by recording start/finish times for each. Videos were analysed by an independent research assistant (ALE), blinded to the infant’s clinical history and knowledge of who was completing the cares or assessments. HR, SpO2, plethysmograph wave, signal identification and quality indicator (signal IQ) and alarm message data were extracted at 5 s intervals during the assessment/care by ALE, and entered into a Microsoft Excel spreadsheet by LGA. During data extraction, motion artefact was determined visually by viewing the pulse amplitude indicator or signal IQ. Only data with good plethysmograph wave and good signal quality with no alarm message (low signal IQ, low perfusion, sensor off or ambient light) were included in the analysis.
The need for nasal continuous positive airway pressure (nCPAP) at the time of clustered nursing cares and neurobehavioural assessments was also recorded as it was considered to be a potential confounding variable that might influence the infant’s tolerance to handling, particularly as it was sometimes removed to allow the infant to move freely during recording of the GMs.
The main outcomes of interest were HR measured as beats per minute (bpm) and SpO2 (%). The occurrence of HR instability, including tachycardia (HR>180 bpm20 21) and bradycardia (HR<100 bpm), was also of interest, as was oxygen desaturation, defined as SpO2<90%.
A sample size of 34 infants was required to find a difference in means between clustered nursing cares and standardised neurobehavioural assessments on a two-sided paired t-test of 0.5 SD and greater, with 81% power and a type-I error of 0.05 assuming one observation for each assessment type per participant.
Statistical analysis
Stata V.13 was used to analyse the data.22 HR and SpO2 were compared between clustered nursing cares and standardised neurobehavioural assessments using linear regression, fitted using generalised estimating equations (GEE) to allow for multiple observations within individual participants. Results are presented as mean differences, 95% CIs and P values. The occurrence of physiological instability for HR and SpO2 was compared between assessment types using logistic regression fitted using GEEs. Results are presented as ORs, 95% CIs and P values. The same outcomes were compared between the standardised neurobehavioural assessments that required handling (PN and HNNE) and the one that did not (GMs), using similar linear and logistic regression models. Analyses were repeated adjusting for the use of nCPAP during clustered nursing cares and standardised neurobehavioural assessments and for the protocol order of cares versus assessments.
Results
Among 143 very preterm infants recruited for the serial neurobehaviour study, 398 video recordings of clustered nursing cares and standardised neurobehavioural assessments were captured between 29 and 32 weeks’ postmenstrual age. Thirty-four eligible videos were randomly selected from 34 individual participants (figure 1). Within these videos, there were 4241 measures of HR and 4246 measures of SpO2 that were included in the analysis. Each infant had between 112 and 293 measurements of HR and SpO2 collected and between 33 and 263 analysed after removing ineligible readings. In 29 of the 34 infants, clustered nursing cares preceded the neurobehavioural assessments.
Figure 1.
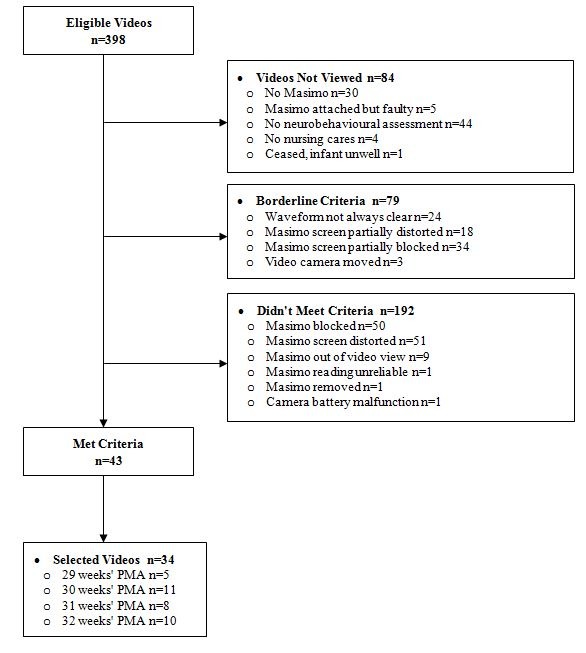
Flow diagram for sample selection. PMA, postmenstrual age.
The mean gestational age at birth of the 34 participants was 27.6 weeks (SD 1.4, range 24–29 weeks) and the mean postmenstrual age at the time of video recording was 30.7 weeks (SD 1.1) (table 1). Thirty-eight per cent of participants were on nCPAP throughout the assessments/cares.
Table 1.
Very preterm infant characteristics
| Characteristics | VP infants n=34 Mean (SD) |
Median (IQR) |
| PMA at assessment (weeks) | 30.7 (1.1) | 31 (30–32) |
| Gestational age at birth (weeks) | 27.6 (1.4) | 27.8 (26.6–28.7) |
| Birth weight (g) | 1071 (225) | 1031 (896–1220) |
| n (%) | ||
| Birth weight ≤2 SD | 1 (3) | |
| Male | 16 (47) | |
| Multiple births | 14 (41) | |
| Maternal antenatal corticosteroids | 30 (88) | |
| Postnatal corticosteroids | 0/26 (0) | |
| Bronchopulmonary dysplasia* | 11 (32) | |
| Necrotising enterocolitis | 1 (3) | |
| Sepsis | 16 (47) | |
| Intraventricular haemorrhage grade I/II | 9 (26) | |
| Intraventricular haemorrhage grade III/IV | 1 (3) | |
| Cystic periventricular leukomalacia | 0 (0) | |
| Surgery prior to hospital discharge | 0 (0) | |
| Higher social risk | 17/30 (57) |
IQR, 25th–75th centiles; median, 50th centile.
*Oxygen dependent at 36 weeks' PMA.
PMA, postmenstrual age; VP, very preterm.
Standardised neurobehavioural assessments versus clustered nursing care
The distributions of HR and SpO2 during standardised neurobehavioural assessments and clustered nursing cares are shown in figure 2; most observations were within expected ranges defined in the Methods section, but there were instances of tachycardia, bradycardia and desaturation in both groups. On average, neurobehavioural assessments were associated with a lower mean HR compared with clustered nursing cares (mean difference −5.9 bpm, 95% CI −6.5 to –5.3, P<0.001), with similar results following adjustment for nCPAP (adjusted mean difference −5.9; 95% CI −6.5 to –5.3; P<0.001) and protocol order (adjusted mean difference −5.9 bpm, 95% CI −6.5 to –5.3, P<0.001). SpO2 was 2.4% (95% CI 2.1 to 2.7; P<0.001) higher on average during neurobehavioural assessments compared with clustered nursing cares, with little effect after adjusting for nCPAP (adjusted mean difference 2.5%; 95% CI 2.1 to 2.8; P<0.001) or protocol order (adjusted mean difference 2.5%; 95% CI 2.1 to 2.8; P<0.001).
Figure 2.
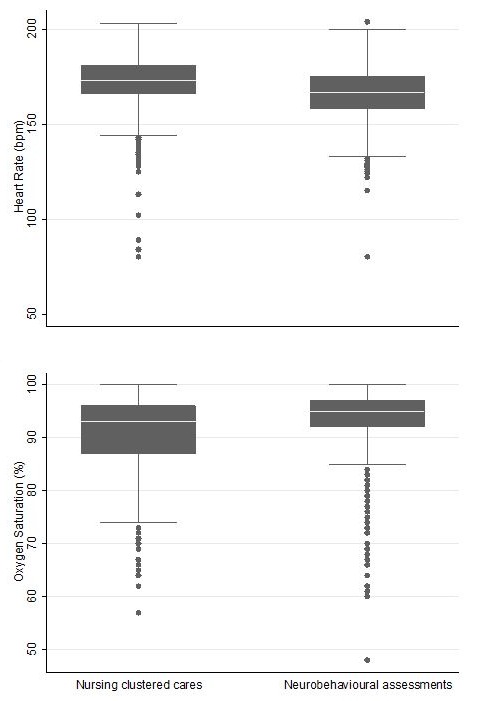
Heart rate and oxygen saturation mean values for standardised neurobehavioural assessments and clustered nursing cares. Median is the solid white line within the box, the 25th and 75th centiles are represented by the margins of the box, and the whiskers represent the range of the data, up to 1.5 times the width of the box, with outliers beyond those ranges represented by separate points.
Neurobehavioural assessments were associated with reduced odds of tachycardia, HR instability and desaturations compared with clustered nursing cares (table 2), even after adjusting for nCPAP and protocol order.
Table 2.
Physiological instability during standardised neurobehavioural assessments and clustered nursing cares
| Physiological instability | Assessment N=2565 n (%) |
Cares N=1693 n (%) |
OR 95% CI |
P value | Adjusted OR* 95% CI |
P value | Adjusted OR† 95% CI |
P value |
| Tachycardia (>180 bpm) |
320 (12) | 453 (27) |
0.44
(0.22 to 0.86) |
0.017 |
0.42
(0.20 to 0.92) |
0.029 |
0.38
(0.20 to 0.74) |
0.005 |
| Bradycardia (<100 bpm) |
1 (0.04) | 3 (0.18) | 0.19 (0.01 to 2.81) |
0.227 | 0.16 (0.01 to 2.02) |
0.158 | 0.16 (0.01 to 2.03) |
0.160 |
| HR instability (>180 or <100 bpm) | 321 (13) | 456 (27) |
0.43
(0.22 to 0.85) |
0.015 |
0.42
(0.20 to 0.90) |
0.026 |
0.37
(0.19 to 0.73) |
0.004 |
| Desaturation (<90%) | 395 (15) | 518 (31) |
0.43
(0.26 to 0.70) |
0.001 |
0.43
(0.26 to 0.70) |
0.001 |
0.43
(0.26 to 0.71) |
0.001 |
*Adjusted for nasal continuous positive airway pressure.
†Adjusted for protocol order.
bpm, beats per minute; HR, heart rate.
There were few episodes of bradycardia associated with either clustered nursing cares or standardised neurobehavioural assessments. There were no serious desaturation episodes requiring resuscitation with either clustered nursing cares or standardised neurobehavioural assessments.
Comparison between standardised neurobehavioural assessments that do and do not require handling
There was strong evidence that on average, HR was higher during assessments that required handling (PN and HNNE; bpm, mean 168.7, SD 11.5) compared with the assessment not requiring handling (GMs; bpm, mean 165.2, SD 13.1; mean difference 5.0 bpm, 95% CI 4.3 to 5.7, P<0.001). Results were unchanged when analyses were adjusted for nCPAP (adjusted mean difference 5.0 bpm; 95% CI 4.3 to 5.7; P<0.001).
The SpO2 was marginally higher during standardised neurobehavioural assessments that required handling (mean 94.3, SD 4.0) compared with the standardised neurobehavioural assessment not requiring handling (mean 93.5, SD 6.2, mean difference 0.4%, 95% CI 0.0% to 0.8%; P=0.046). Results remained similar when analyses were adjusted for nCPAP (adjusted mean difference 0.4; 95% CI 0.0% to 0.8%; P=0.043). Rates of tachycardia (handling 15%, no handling 11%), bradycardia (handling 0%, no handling 0.1%), HR instability (handling 15%, no handling 12%) and desaturations (handling 11%, no handling 18%) were low and similar in both groups.
There was evidence that assessments requiring handling were associated with an increased odds of tachycardia (OR 1.76; 95% CI 1.08% to 2.86%; P=0.024) and HR instability (OR 1.75; 95% CI 1.08% to 2.85%; P=0.024) compared with the non-handling assessment. Conclusions were unchanged when analyses were adjusted for nCPAP.
Discussion
In the current study, physiological stability appeared to be better maintained during standardised neurobehavioural assessments than during clustered nursing cares, with a lower average HR, and lower odds of tachycardia, HR instability and desaturation. Conclusions were unaltered when analyses were adjusted for treatment with nCPAP or protocol order. There was also evidence that HR was on average higher during standardised neurobehavioural assessments that required handling (PN 1 and HNNE) compared with assessments not requiring handling (GMs).
There have been no previous studies of physiological stability comparing standardised neurobehavioural assessments with clustered nursing cares with which to compare the results of our study. Several studies have reported increased HR during clustered nursing care procedures. One study measured behavioural state, facial activity and HR in 48 infants undergoing a heel prick procedure within the first 4 days of life (21 infants were handled prior compared with 27 infants not handled prior to the procedure).23 They found evidence that the HR during the procedure was increased compared with the baseline measurement, with a 30.5 (SD 14.9) bpm mean HR increase for the handled group and a 22.6 (SD 15.8) bpm mean HR increase for the non-handled group. Another study explored changes in HR in 37 infants during nursing cares divided into five levels of stress (1=sound or light; 2=sound and light; 3=sound or light and handling; 4=sound, light and handling; 5=any intervention that causes pain); HR was increased with nursing cares 2, 3, 4 and 5 compared with level 1.24 Two studies have reported increased HR during neurobehavioural/neurological assessments. The first study reported increased tachycardia in very preterm infants with bronchopulmonary dysplasia (BPD) compared with infants without BPD (P=0.01) at 31–38 weeks’ postmenstrual age undergoing weekly neurological assessment adapted from Dubowitz and Dubowitz neurobehavioural assessment.25 26 However, this study was conducted in 1988 and respiratory support for preterm infants has changed considerably since then. The second study reported increased HR during the neurological assessment of the preterm and full-term newborn infant19 in 36 preterm infants (30–35 weeks' gestation) compared with 36 full-term infants (39–41 weeks' gestation) (P<0.001).2
Of interest in the current study was the low rate of bradycardia during both clustered nursing cares (one occurrence during a nasogastric tube change, and two occurrences back to back during a temperature recording) and standardised neurobehavioural assessments (one occurrence during GMs assessment). A study of 32 preterm infants born at 24–32 weeks' gestational age found evidence of more bradycardia during a weekly neurobehavioural assessment in the infants with BPD, compared with the infants without BPD (P<0.001).25 Although perinatal variables were not formally analysed in the current study, BPD did not affect rates of bradycardia given the only infant to have a bradycardia during neurobehavioural assessment did not have BPD.
Higher HR during assessments requiring handling compared with no handling has been reported by others. For example, one study compared HR during items requiring handling, such as head lag with pull to sit, ventral suspension and stepping reflex, compared with items requiring little or no handling, for example, orientation and movement observation.2
The current study is the first to report that standardised neurobehavioural assessments were associated with reduced odds of desaturations compared with clustered nursing cares, and it is the first to report SpO2 during individual standardised neurobehavioural assessments. Mean SpO2 was higher for standardised neurobehavioural assessments that require handling compared with those that did not (GMs), which was unexpected because other studies have reported that increased handling causes decreases in SpO2.4 24 27 An explanation for the difference could be that GMs are filmed with the infants supine. As very preterm infants are usually positioned in prone or side-lying for respiratory support, they may not have been able to tolerate the change in position to supine for the duration of filming after already having been in supine for the nursing cares. Alternatively, the increased rate of desaturations may also be because GMs were completed first and when nCPAP was initially stopped. Regardless, the size of the mean difference in SpO2 of 0.4% was trivial clinically.
The current study has a number of strengths. The large volume of data for both HR and SpO2 meant that the study had ample power to compare these important outcomes between neurobehavioural assessments and nursing cares. The perinatal characteristics of our cohort, with the presence of morbidities such as necrotising enterocolitis, intraventricular haemorrhage, sepsis and oxygen dependency at 36 weeks, are typical of infants born <30 weeks’ gestation so that our results should be generalisable to similar settings. The weaknesses of the study include that the order of nursing cares and neurobehavioural assessments could not be randomly allocated because clinical needs took priority over research. Nonetheless, this reflects clinical practice and we would recommend that nursing cares are performed prior to neurobehavioural assessments in the NICU in order of priority. Also, although some results reached statistical significance they may not be clinically important.
Given current literature in this area combines both physiological responses and behavioural cues to evaluate infant stress, future research should include a study designed to evaluate both of these in relation to a comparison of clustered nursing cares and standardised neurobehavioural assessments. Moreover, comparisons of individual neurobehavioural items, individual components of nursing care and techniques used to support infant stability (eg, hand containment and pacing) are also warranted.
Conclusion
In conclusion, this study demonstrates that standardised neurobehavioural assessments were associated with less physiological stress than clustered nursing cares in infants aged 29–32 weeks' postmenstrual age, and are therefore possible to complete without causing undue physiological disturbance in medically stable infants.
Acknowledgments
We would like to acknowledge the VIBeS team who contributed to recruitment and data collection, and the staff at the Royal Women’s Hospital, Melbourne NICU and SCN who contributed to data collection.
Footnotes
Contributors: LWD and AJS conceptualised and designed the study with study design assistance from LD and JAD. LGA and ALE were responsible for acquisition of data. LGA carried out data analyses with the assistance of KJL, LWD and AJS who provided interpretation of analysed data. LGA drafted the initial manuscript. LGA, LWD, AJS, LD, JAD, ALE and KJL reviewed, revised and approved the final manuscript.
Funding: This work was supported in part by the Australian National Health and Medical Research Council (Project Grant ID 1024516); Centre of Clinical Research Excellence (Grant ID 546519); Centre of Research Excellence Grant (ID 1060733); Early Career Fellowship (ID 1053767) to AJS; Career Development Fellowships to AJS (ID 1108714) and KJL (ID1053609); Australian Postgraduate Scholarship to LGA; the Victorian Government Operational Infrastructure Support Program; and The Royal Children’s Hospital Foundation.
Competing interests: AJS is a member of the General Movements Trust, a not-for-profit organisation, involved in training the General Movements Assessment. The other authors have no potential conflicts of interest relevant to this article to disclose.
Ethics approval: Human Research Ethics Committees at the Royal Women’s Hospital, Melbourne.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There are no additional unpublished data from the study to share.
References
- 1. Anand KJ, Carr DB. The neuroanatomy, neurophysiology, and neurochemistry of pain, stress, and analgesia in newborns and children. Pediatr Clin North Am 1989;36:795–822. doi:10.1016/S0031-3955(16)36722-0 [DOI] [PubMed] [Google Scholar]
- 2. Sweeney JK, Blackburn S. Neonatal physiological and behavioral stress during neurological assessment. J Perinat Neonatal Nurs 2013;27:242–52. doi:10.1097/JPN.0b013e31829dc329 [DOI] [PubMed] [Google Scholar]
- 3. Holsti L, Grunau RE, Oberlander TF, et al. Prior pain induces heightened motor responses during clustered care in preterm infants in the NICU. Early Hum Dev 2005;81:293–302. doi:10.1016/j.earlhumdev.2004.08.002 [DOI] [PubMed] [Google Scholar]
- 4. Johnston CC, Stevens BJ. Experience in a neonatal intensive care unit affects pain response. Pediatrics 1996;98:925–30. [PubMed] [Google Scholar]
- 5. Salavitabar A, Haidet KK, Adkins CS, et al. Preterm infants’ sympathetic arousal and associated behavioral responses to sound stimuli in the neonatal intensive care unit. Adv Neonatal Care 2010;10:158–66. doi:10.1097/ANC.0b013e3181dd6dea [DOI] [PubMed] [Google Scholar]
- 6. Brown G. NICU noise and the preterm infant. Neonatal Netw 2009;28:165–73. doi:10.1891/0730-0832.28.3.165 [DOI] [PubMed] [Google Scholar]
- 7. Bremmer P, Byers JF, Kiehl E. Noise and the premature infant: physiological effects and practice implications. J Obstet Gynecol Neonatal Nurs 2003;32:447–54. doi:10.1177/0884217503255009 [DOI] [PubMed] [Google Scholar]
- 8. Craig KD, Whitfield MF, Grunau RV, et al. Pain in the preterm neonate: behavioural and physiological indices. Pain 1993;52:287–99. doi:10.1016/0304-3959(93)90162-I [DOI] [PubMed] [Google Scholar]
- 9. Wadhwa PD, Culhane JF, Rauh V, et al. Stress, infection and preterm birth: a biobehavioural perspective. Paediatr Perinat Epidemiol 2001;15(Suppl 2):17–29. doi:10.1046/j.1365-3016.2001.00005.x [DOI] [PubMed] [Google Scholar]
- 10. Johnson AN. Adapting the neonatal intensive care environment to decrease noise. J Perinat Neonatal Nurs 2003;17:280–8. doi:10.1097/00005237-200310000-00006 [DOI] [PubMed] [Google Scholar]
- 11. Johnston CC, Stevens BJ, Yang F, et al. Differential response to pain by very premature neonates. Pain 1995;61:471–9. doi:10.1016/0304-3959(94)00213-X [DOI] [PubMed] [Google Scholar]
- 12. Peng NH, Chen CH, Bachman J, et al. To explore relationships between physiological stress signals and stress behaviors in preterm infants during periods of exposure to environmental stress in the hospital. Biol Res Nurs 2011;13:357–63. doi:10.1177/1099800410392020 [DOI] [PubMed] [Google Scholar]
- 13. Holsti L, Grunau RE, Oberlander TF, et al. Body movements: an important additional factor in discriminating pain from stress in preterm infants. Clin J Pain 2005;21:491–8. doi:10.1097/01.ajp.0000146163.30776.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–9. doi:10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 15. Vandenbroucke JP,et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med 2007;147:W163–194. doi:10.7326/0003-4819-147-8-200710160-00010-w1 [DOI] [PubMed] [Google Scholar]
- 16. Spittle AJ, Thompson DK, Brown NC, et al. Neurobehaviour between birth and 40 weeks' gestation in infants born <30 weeks’ gestation and parental psychological wellbeing: predictors of brain development and child outcomes. BMC Pediatr 2014;14:111 doi:10.1186/1471-2431-14-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prechtl HF. Qualitative changes of spontaneous movements in fetus and preterm infant are a marker of neurological dysfunction. Early Hum Dev 1990;23:151–8. doi:10.1016/0378-3782(90)90011-7 [DOI] [PubMed] [Google Scholar]
- 18. Daily DK, Ellison PH. The premie-neuro: a clinical neurologic examination of premature infants. Neonatal Netw 2005;24:15–22. doi:10.1891/0730-0832.24.1.15 [DOI] [PubMed] [Google Scholar]
- 19. Dubowitz LMS, Dubowitz V, Mercuri E. The neurological assessment of the preterm and full-term newborn infant Clinics in developmental medicine 148, 2nd ed London: MacKeith Press, 1999. [Google Scholar]
- 20. World Health Organization. Integrated management of pregnancy and childbirth: pregnancy, childbirth, postpartum and newborn care; a guide for essential practice. 3rd ed Geneva: World Health Organization, 2003:186. [PubMed] [Google Scholar]
- 21. Haba J. Approach to pediatric tachycardia. Columbia: Department of Pediatrics, University of British Columbia; http://learn.pediatrics.ubc.ca/body-systems/cardiology/approach-to-pediatric-tachycardia/. [Google Scholar]
- 22. StataCorp LP. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP, 2013. [Google Scholar]
- 23. Porter FL, Wolf CM, Miller JP. The effect of handling and immobilization on the response to acute pain in newborn infants. Pediatrics 1998;102:1383–9. doi:10.1542/peds.102.6.1383 [DOI] [PubMed] [Google Scholar]
- 24. Peng NH, Bachman J, Jenkins R, et al. Relationships between environmental stressors and stress biobehavioral responses of preterm infants in NICU. J Perinat Neonatal Nurs 2009;23:363–71. doi:10.1097/JPN.0b013e3181bdd3fd [DOI] [PubMed] [Google Scholar]
- 25. Medoff-Cooper B. The effects of handling on preterm infants with bronchopulmonary dysplasia. Image J Nurs Sch 1988;20:132–4. doi:10.1111/j.1547-5069.1988.tb00052.x [DOI] [PubMed] [Google Scholar]
- 26. Dubowitz L, Dubowitz V. The neurological assessment of the preterm and full-term newborn infant. clinics in developmental medicine 79, 1 London, England: MacKeith Press, 1981. [Google Scholar]
- 27. Zahr LK, Balian S. Responses of premature infants to routine nursing interventions and noise in the NICU. Nurs Res 1995;44:179–85. doi:10.1097/00006199-199505000-00009 [PubMed] [Google Scholar]


