Abstract
Aims
Despite the success of statins, there remains unmet clinical need in cardiovascular disease (CVD) prevention. New proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors reduce low-density lipoprotein cholesterol (LDL-C) by 55–65%. Two PCSK9 inhibitors, evolocumab, and alirocumab, were approved for use in Norway but not yet for reimbursement through public national insurance. We aim to explore the cost-effectiveness of these compared with available treatments in a Norwegian setting.
Methods and results
A state transition Markov model was developed to model the cost-effectiveness of PCSK9 inhibitors for prevention of coronary heart disease, ischaemic strokes, and death among high-risk patient subpopulations in Norway, in both primary and secondary settings. Evolocumab and alirocumab are compared against ezetimibe and standard treatment. Risk of CVD is based on population incidence rates and adjusted according to baseline risk factors. Preventative effect of treatment was modelled according to absolute reduction in LDL-C. PCSK9 inhibitors were never found to be cost-effective in primary prevention. In secondary prevention they were cost-effective only for older, high-risk patients. The lowest cost-effectiveness ratios were for heterozygous familial hypercholesterolaemia patients and high-risk diabetics, with €63 200 and €68 400 per quality-adjusted life-year, respectively.
Conclusion
High lifetime costs of PCSK9 inhibitors may not be offset by estimated health gains for most eligible patients. PCSK9 inhibitors are found in the model only to be cost-effective in secondary prevention for older patients with high absolute risk of CVD. This picture is likely to change as price decreases. Future research is needed to determine the long-term preventative effects of PCSK9 inhibitors.
Keywords: Cost-effectiveness, Cost-utility, PCSK9, Evolocumab, Alirocumab, Economic evaluation
Introduction
A high level of low-density lipoprotein cholesterol (LDL-C) is a well-documented risk factor for the development of CVD and the incidence of acute cardiac events. LDL-C is a modifiable factor, the lowering of which can lead to decreased risk of CVD.1
Individuals who are at risk for CVD events are recommended high-intensity LDL-C reduction treatment therapies.2,3 Despite widespread use and success of statins in the reduction of LDL-C and prevention of CVD, however, there remain unmet clinical needs in achieving LDL-C reduction goals.4,5 This includes individuals with heterozygous familial hypercholesterolaemia (HeFH), statin intolerance, and other high-risk patients who are not meeting target goals for lipid reduction with statins and lifestyle changes alone—particularly diabetics and those who have previously experienced CVD events.3,6,7 Ezetimibe has been used with some success as a next-line-of-defence cholesterol treatment, though its use and the extent it addresses unmet need is limited.8,9
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors evolocumab and alirocumab have recently been approved for use as lipid modifying therapies in both the US and Europe. Both drugs have been demonstrated to be safe, well tolerated, and extremely effective at lowering concentrations of LDL-C in the blood—in most cases by 55–65%.10,11 Cost-effectiveness of these drugs has not yet been well established.5 Several analyses out of the US suggest that PCSK9 inhibitors are not cost-effective, though at least one has found that they are.4,12–14 They are significantly less expensive in Europe, however, which means PCSK9 inhibitors may be cost-effective for other countries, including Norway. The UK’s National Institute for Health and Care Excellence (NICE) has recently approved it for limited use in some high-risk patient groups.15 In Norway, PCSK9 inhibitors are only reimbursed for homozygous familial hypercholesterolaemia patients, which are approximately 10 persons in the whole country. Current unmet clinical needs in LDL-C reduction reflect the candidate populations who are targets for PCSK9 inhibitors. Randomized control trials show substantial LDL-C reductions with PCSK9 inhibitors for all patients.10,11
The objective of this article is to develop a state-transition Markov model in Microsoft Excel in order to model the cost-effectiveness of PCSK9 inhibition for the prevention of cardiovascular events and CVD in Norway. The model specifically addresses PCSK9 inhibitors as compared with ezetimibe, and focuses on high-risk individuals.
Methods
Model structure and input
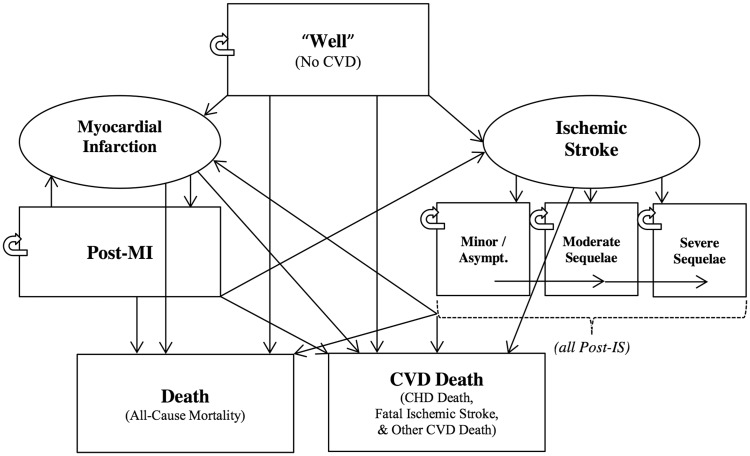
A state-transition Markov model was developed to model the incidence of atherosclerotic cardiovascular disease events—specifically myocardial infarction (MI), ischaemic stroke (IS), and death. The model estimates incidence of CVD specifically within the Norwegian population for both primary and secondary prevention settings. Individuals who are ‘well’ are at risk for experiencing first-ever CVD events; those who survive these events transition into chronic post-CVD health states, where they remain at heightened risk for further events or death (Figure 1). ‘Well’ only indicates the absence of previous CVD events—these individuals may still be at a very high-risk for CVD due to various other baseline risk factors.
Figure 1.
Simplified schematic of state transition Markov model. Rectangles represent health states while ovals represent events. Patients can move in the direction of any arrow. Patients can be in only one health state per cycle.
A cohort of patients can begin the model at any age from 30 years upward and runs up to age 100 or until everyone is dead. Men and women can be modelled in separate cohorts or combined. Baseline risk factors can be adjusted upwards to reflect heightened risk, while LDL-C reduction treatments reduce baseline risk according to the absolute measure of LDL-C reduction achieved.
Absolute reductions in baseline LDL-C as a result of treatment were taken from meta-analyses of PCSK9 inhibitor RCTs (Table 1). Subsequent relative risk reductions are modelled according to CTT meta-analyses of the effect of LDL-C reductions on CVD risk (Table 1).
Table 1.
Key treatment effect parameters
| Relative Risks (per mmol/L LDL-C reduction) | RR | (SE) | Source |
|---|---|---|---|
| Non-fatal MI | 0.74 | (0.03) | 1,16 |
| CHD Death | 0.81 | (0.01) | 1,16 |
| Non-fatal IS | 0.81 | (0.05) | 1,16 |
| Fatal IS | 0.91 | (0.10) | 1,16 |
| Other CVD Death | 0.95 | (0.10) | 1,16 |
| % LDL-C Reductions from Baseline | % Change | (SE) | Source |
|---|---|---|---|
| Evolocumab | 0.63 | (0.01) | 11 |
| Alirocumab | 0.56 | (0.01) | 11 |
| Ezetimibe | 0.24 | (0.01) | 11 |
LDL-C reductions from PCSK9 inhibitors and effects of LDL-C reduction on CVD risk.
Individuals who experience non-fatal CVD events and transition from the primary to the secondary component of the model are at increased risk for experiencing additional CVD events or death. Relative risks for those in chronic post-CVD states are taken from a variety of sources (see Supplementary material online, Appendix S1).
The setting of this analysis is the Norwegian healthcare sector, which is publicly financed by the Norwegian National Health Insurance scheme and as such the focus is on direct medical costs.17
A lifetime horizon was chosen for this analysis. Initiation of PCSK9 inhibitors or ezetimibe is considered here to be the initiation of lifelong treatment.
A discount rate of 4% is used for both future costs and utilities, as suggested by the Norwegian Ministry of Finance.18 Costs and results were converted from 2015 Norwegian Kroner (NOK) to 2015 Euros (€) for presentation in this report.
A cost-effectiveness threshold of 600 000 NOK (€67 165) per additional QALY gained is typical for Norwegian economic evaluations. Though this is widely used, it is an unofficial guideline rather than a strict rule. Treatments and drugs with ICERs higher than this may also be approved for reimbursement in Norway.19
Quality of life
The primary health outcome of this analysis is the Quality-Adjusted Life Year (QALY). We used QALY weights based on the EQ-5D HRQoL questionnaire and Time-Trade-Off (TTO) methods and valuation based on the UK tariff. QALY values are assigned to all chronic CVD states as well as healthy, non-CVD states (see Supplementary material online, Appendix S1). Use of the same tariff for all values helps to maintain consistency across QALY estimates.20
Resource use and costs
Resource-use is estimated for CVD events and health outcomes; most estimates are made according to methods described in the Norwegian Cardiovascular Disease Model (NorCaD).18 NorCaD costs are well validated, and are frequently cited in Norwegian economic evaluations and health technology assessments.21–25 Costs assigned to each individual resource or cost component are taken from publicly available information.26–29
Evolocumab and alirocumab are each administered as injections every two weeks. Evolocumab costs €1813.56 for six injections; the cost for 1 year (52 weeks) is €7858.13. Alirocumab costs €1783.61 for six injections; 1 year costs €7728.38.27
Sensitivity analysis
Scenario analysis was performed on price of evolocumab and alirocumab, reducing these by 50%.
Probabilistic sensitivity analysis (PSA) was undertaken according to methods laid out by Briggs et al. The model was simulated 1000 times using random draws for each input parameter according to its respective distribution; this provides the probabilistic output of the model and a clearer picture of the uncertainty surrounding point estimates and mean output. Probabilistic output is recorded and analysed within the net benefits framework, and presented as Cost-Effectiveness Acceptability Curves (CEAC).17
Expected Value of Perfect Information (EVPI) analysis was performed to estimate the value of reducing uncertainty through new research or more information; EVPI estimates are based on PSA output. Analysis of the Expected Value of Perfect Information for Parameters (EVPPI) was also undertaken to determine for which specific parameters reduced uncertainty would yield the highest estimated value of new research.17
Characteristics of patients
We assumed very high-risk patients would be prime initial candidates for treatment. Modelling sometimes necessitates both assumptions and limitations in terms of what is modelled, as incorporating all possible variation and complexity is impossible. Our objective was to test a wide range of risk levels across age groups in both primary and secondary prevention, so we limited our focus to four base high-risk clinical profiles (Table 2). These profiles represent high-risk patients from candidate populations with unmet clinical need. Baseline characteristics come partially from characteristics of patients in RCTs, partially from diagnostic criteria, and partially from assumption. Primary prevention is defined here as prevention for those who have never suffered a previous myocardial infarction or ischaemic stroke. Secondary prevention focuses on patients who have previously suffered myocardial infarctions.
Table 2.
Patient profiles and baseline risk factors
| No. | Description | Total Cholesterol (mmol/L) | LDL-C (mmol/L) | Hypertension (SBP mm/Hg) | Diabetes | Smoker |
|---|---|---|---|---|---|---|
| 1 | Diabetic | 6.2 | 3.9 | Y (145) | Y | N |
| 2 | HeFH | 9.2 | 6.2 | N | N | N |
| 3 | Statin Intolerant | 7.3 | 4.9 | N | N | N |
| 4 | Misc. High Risk | 6.5 | 4.0 | Y (145) | N | N |
‘N’ denotes ‘No’ and ‘Y’ denotes ‘Yes’ to indicate respectively the absence or presence of a risk factor. SBP denotes systolic blood pressure and is listed for hypertensive patients only. HeFH indicates heterozygous familial hypercholesterolaemia.
Results
Primary prevention
Ezetimibe, alirocumab, and evolocumab each lead to QALY gains across all four patient groups (Table 3). HeFH patients see the biggest gain in QALYs (discounted), from 8.74 with only statin therapy, to 9.50 when ezetimibe is added, and increasing again 10.34 with evolocumab. QALYs for diabetic patients are 7.31 with statins and increase to 7.83 with the addition of ezetimibe, and 8.57 with the addition of evolocumab. Monotherapy for statin intolerant patients sees a slightly smaller gain, increasing from 9.90 with no treatment to 10.29 QALYs with ezetimibe, and then to 10.95 with evolocumab. QALY gains for the miscellaneous high-risk group are slightly smaller. Alirocumab leads to similar but slightly smaller health gains than evolocumab for all patient groups.
Table 3.
Primary prevention for 65 year-olds (all numbers discounted and per person)
| Drug cost (€) | CVD cost (€) | QALYs | ICER (Δ€/ΔQALY) | |
|---|---|---|---|---|
| Diabetics | ||||
| Standard | — | 46 905 | 7.31 | — |
| Ezetimibe | 5001 | 43 836 | 7.83 | 3716 |
| Alirocumab | 78 377 | 38 709 | 8.45 | Dominated |
| Evolocumab | 80 879 | 37 498 | 8.57 | 93 938 |
| HeFH | ||||
| Standard | — | 24 583 | 8.74 | — |
| Ezetimibe | 6092 | 20 283 | 9.50 | 2369 |
| Alirocumab | 95 229 | 14 692 | 10.21 | Dominated |
| Evolocumab | 98 007 | 13 609 | 10.34 | 101 351 |
| Statin Intolerant | ||||
| Standard | — | 21 796 | 9.90 | — |
| Ezetimibe | 6635 | 19 187 | 10.29 | 10 505 |
| Alirocumab | 101 796 | 14 328 | 10.86 | Dominated |
| Evolocumab | 104 357 | 13 495 | 10.95 | 138 943 |
| Misc. High Risk | ||||
| Standard | — | 20 897 | 10.33 | — |
| Ezetimibe | 6902 | 18 238 | 10.67 | 12 170 |
| Alirocumab | 103 773 | 14 848 | 11.05 | Dominated |
| Evolocumab | 106 208 | 14 152 | 11.12 | 212 700 |
Primary prevention indicates that patients have no history of myocardial infarction or ischaemic stroke. Standard treatment reflects whatever statin regimen the patients were on prior to initiation of PCSK9 or ezetimibe therapy. No drug cost was used for standard treatment; it was assumed that statin regimens would not change according to treatment and would therefore have no bearing on an incremental comparison of costs.
The cost per patient of treating manifest CVD decreases with each incremental treatment for all patient groups. For diabetic patients costs decrease from €46 905 on standard treatment to €43 836 with ezetimibe, and then drop again to €37 498 with evolocumab. Decreases in CVD costs for the other three patient costs are comparable. Alirocumab results in slightly less CVD costs saved than evolocumab for all patient groups.
Increases in lifetime drug costs per patient are quite substantial with both PCSK9 inhibitors. Ezetimibe drug costs range from €5000 to €6900, while alirocumab is €78 000 to €103 000 and evolocumab is €81 000 to €106 000. Alirocumab is €2000–€3000 less expensive than evolocumab for all patient groups at current prices.
Increments in costs divided by increments in QALYs, ICERs are for PCSK9 inhibitors amongst 65 year-olds in the range of €94 000–€213 000 per QALY gained in primary prevention. For 50 year-old patient groups, ICERs were hundreds of thousands of Euros per QALY; when analysis is stratified by gender, men have consistently lower ICERs than women (see Supplementary material online, Appendix S2).
Secondary prevention
QALYs are somewhat lower in secondary prevention due to the increased risk in these groups. QALY gains, however, are similar to those observed in primary prevention for all treatments, and in some cases slightly larger (Table 4). Treatment costs are generally higher among secondary compared with primary prevention patients, while treatment drug costs are somewhat lower.
Table 4.
Secondary prevention for 65 year-olds (all numbers per person)
| Drug cost (€) | CVD cost (€) | QALYs | ICER (Δ€/ΔQALY) | |
|---|---|---|---|---|
| Diabetics | ||||
| Standard | — | 64 872 | 4.67 | — |
| Ezetimibe | 3701 | 64 816 | 5.22 | 6544 |
| Alirocumab | 60 937 | 62 348 | 5.91 | Dominated |
| Evolocumab | 63 468 | 61 495 | 6.05 | 68 386 |
| HeFH | ||||
| Standard | — | 37 679 | 5.85 | — |
| Ezetimibe | 4863 | 35 489 | 6.85 | 2654 |
| Alirocumab | 81 406 | 30 036 | 7.84 | Dominated |
| Evolocumab | 84 646 | 28 695 | 8.01 | 63 174 |
| Statin Intolerant | ||||
| Standard | — | 38 106 | 7.07 | — |
| Ezetimibe | 5459 | 36 237 | 7.62 | 6588 |
| Alirocumab | 88 304 | 31 016 | 8.44 | Dominated |
| Evolocumab | 91 176 | 29 923 | 8.56 | 84 428 |
| Misc. High Risk | ||||
| Standard | — | 38 594 | 7.55 | — |
| Ezetimibe | 5799 | 36 311 | 8.05 | 6969 |
| Alirocumab | 90 182 | 32 439 | 8.59 | Dominated |
| Evolocumab | 92 841 | 31 525 | 8.69 | 128 191 |
Secondary prevention indicates all patients have a history of myocardial infarction.
Standard treatment reflects whatever statin regimen the patients were on prior to initiation of PCSK9 or ezetimibe therapy. No drug cost was used for standard treatment; it was assumed that statin regimens would not change according to treatment and would therefore have no bearing on an incremental comparison of costs.
PCSK9 inhibitors are cost-effective for the HeFH patient group at 65 years of age with an ICER of €63 174/QALY. Use of PCSK9 inhibitors for 65 year-old diabetics is on the border of cost-effectiveness with an ICER of €68 386/QALY. The ICERs for those aged 70 and older are higher than ICERs for the 65 year-olds for all patient groups, but are still borderline cost-effective for the diabetic and HeFH patient groups. Initiating PCSK9 therapy for those younger than 65 is not cost-effective in any patient group; when results are stratified by gender, ICERs for treating men are once again lower than those for treating women (see Supplementary material online, Appendix S2).
Scenario analyses
With a 50% price reduction, PCSK9 inhibitors are cost-effective for all diabetic and HeFH patients, and cost-effective or borderline for older patients and secondary patients in less severe risk groups (lower section of Table 5).
Table 5.
Most cost-effective alternative across multiple ages and scenario analysis of price
| Diabetic |
HeFH |
Statin intolerant |
Misc. high risk |
|||||
|---|---|---|---|---|---|---|---|---|
| Age | Primary | Secondary | Primary | Secondary | Primary | Secondary | Primary | Secondary |
| Current Market Price (∼€7800 per person, per year): | ||||||||
| 50 | Ezetimibe | Ezetimibe | Ezetimibe | Ezetimibe | Ezetimibe | Ezetimibe | Ezetimibe | Ezetimibe |
| 55 | Ezetimibe | Ezetimibe | Ezetimibe | Ezetimibe | Ezetimibe | Ezetimibe | Ezetimibe | Ezetimibe |
| 60 | Ezetimibe | Ezetimibe | Ezetimibe | Ezetimibe | Ezetimibe | Ezetimibe | Ezetimibe | Ezetimibe |
| 65 | Ezetimibe | Eze/PCSK9* | Ezetimibe | PCSK9 | Ezetimibe | Ezetimibe | Ezetimibe | Ezetimibe |
| 70 | Ezetimibe | Eze/PCSK9* | Ezetimibe | Eze/PCSK9* | Ezetimibe | Ezetimibe | Ezetimibe | Ezetimibe |
| 75 | Ezetimibe | Eze/PCSK9* | Ezetimibe | Eze/PCSK9* | Ezetimibe | Ezetimibe | Ezetimibe | Ezetimibe |
| 50% of Price (∼€3900 per person, per year): | ||||||||
| 50 | PCSK9 | PCSK9 | PCSK9 | PCSK9 | Ezetimibe | Ezetimibe | Ezetimibe | Ezetimibe |
| 55 | PCSK9 | PCSK9 | PCSK9 | PCSK9 | Ezetimibe | PCSK9 | Ezetimibe | Ezetimibe |
| 60 | PCSK9 | PCSK9 | PCSK9 | PCSK9 | Ezetimibe | PCSK9 | Ezetimibe | Eze/PCSK9* |
| 65 | PCSK9 | PCSK9 | PCSK9 | PCSK9 | Eze/PCSK9* | PCSK9 | Ezetimibe | PCSK9 |
| 70 | PCSK9 | PCSK9 | PCSK9 | PCSK9 | Eze/PCSK9* | PCSK9 | Ezetimibe | PCSK9 |
| 75 | PCSK9 | PCSK9 | PCSK9 | PCSK9 | PCSK9 | PCSK9 | Ezetimibe | PCSK9 |
Cells indicate the most cost-effective treatment option for the respective patient/age group at a willingness-to-pay threshold of 600 000 NOK/QALY (€67 165/QALY). Eze/PCSK9* indicates borderline cost-effectiveness, meaning the ICER for PCSK9 inhibitors was between 600 000 and 700 000 NOK/QALY (€67 165–€78 360/QALY).
Probabilistic sensitivity analysis
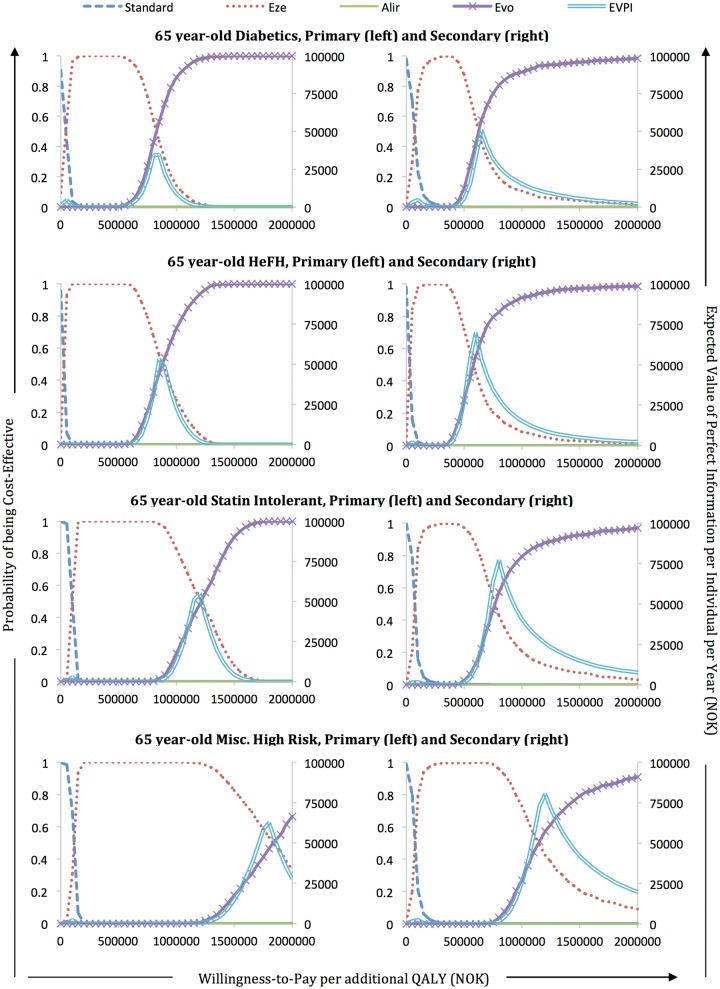
Cost-effectiveness acceptability curves (CEACs) from probabilistic sensitivity analysis indicate that there is a very low probability that PCSK9 inhibitors are cost-effective given a 600 000 NOK/QALY (€67 165/QALY) threshold (Figure 2). Assuming this WTP threshold, the probability that evolocumab is cost-effective in primary prevention is close to or at 0% for all patient groups. The diabetic patient group has the highest probability with 3.4%. In secondary prevention, the probability that evolocumab is cost-effective at this WTP threshold is 55.2% for HeFH patients, 42.0% for diabetic patients, and 13.2% for statin intolerant patients; it is still 0% for the miscellaneous high risk group.
Figure 2.
Cost-effectiveness acceptability curves and expected value of perfect information for 65 year-olds. Standard societal willingness-to-pay threshold is 600 000 NOK (€67 165) per QALY. Outer axis labels apply to each individual graph according to its corresponding position in the overall picture.
EVPPI analysis for 65 year-old HeFH patients at a standard willingness to pay threshold of 600 000 NOK/QALY (€67 165/QALY) suggests most value would come from reducing uncertainty around baseline risk and the treatment effect variables. EVPPI results also indicate value from reducing uncertainty around QALY values, which would likely be resolved by splitting the post-MI health state into several smaller, more specific health states (see Supplementary material online, Appendix S2). For most other patient groups, EVPPI analysis at a WTP threshold of 600 000 NOK (€67 165) yields 0 for all parameters and parameter groups, indicating that there is no uncertainty needed to reduce as long as prices are at current levels.
Discussion
Cost of treatment with PCSK9 inhibitors over many years at current market prices will be extremely expensive. In this analysis, all patient groups are at considerable risk for lifetime CVD.3 Yet despite projections of gains in QALYs, reductions in CVD events and CVD deaths, and incremental cost-savings in terms of treating manifest CVD, our model finds that the benefits of the drugs almost never offset their high long-term costs. Cost-effectiveness was only found likely in older patients with extremely high risk and a history of myocardial infarction.
Cost-effectiveness in our model is therefore very sensitive to long-term price, which is why older patients are found to be more cost-effective. Our modelling results are likely at odds with what many clinicians consider to be the potential clinical value of PCSK9 inhibitors. There is no doubt that preventing a heart attack for a young patient has tremendous clinical value, more so than preventing a heart attack for a much older patient. What our model suggests is that the high cost of PCSK9 inhibitors over many years, if not decades, of treatment may not be offset by the amount of CVD prevented and the clinical value gained, given the current price of the drug and standard willingness-to-pay thresholds. Discounting is also an important consideration when modelling younger patients: if prices are high and QALY gains are further into the future, this will yield a less cost-effective result. Our model is not intended to be a clinical analysis in this sense, but rather an economic one.
Because they are direct comparators in next-line-of-defence pharmacological cholesterol reduction, the total number of patients currently using ezetimibe is likely the best estimation of the wider patient population who might be immediately eligible for PCSK9 inhibitors. Given the massive budget impact if all eligible users were to immediately begin therapy with PCSK9 inhibitors, the results of this modelling exercise can provide some level of insight into which patient populations would be the most cost-effective and should perhaps receive them first.
Our model suggests that as the cost of PCSK9 inhibitors decreases, increasing access should focus on younger high-risk patients rather than moderate-risk older patients (Table 5). Allocation of PCSK9 inhibitors will then fit well with the age-differentiated Norwegian prioritization guidelines as discussed in NORRISK, because treating younger, high-risk patients yields higher expected benefits and is more cost-effective than treating older patients at more moderate levels of risk.30
The fact that the Norwegian willingness-to-pay threshold tends to be flexible between €67 165 and €78 360 (600 000 and 700 000 NOK) per QALY changes the conclusion about cost-effectiveness of use of PCSK9 inhibitors in some patient groups. In Table 5, we showed that 6 groups instead of only 1 was cost-effective if the threshold is €78 360 instead of €67 165.
Our analysis was limited to the 140 mg dose of evolocumab, as this is the only available dose in Norway. For alirocumab we included only the highest available 150 mg dose of alirocumab and did not allow for titration from lower to higher doses; annual cost of alirocumab is not affected by titration in Norway. Our model found evolocumab to be more cost-effective than alirocumab across all patient profiles and age groups. This is most likely to be a result of how the modelling was performed, as CVD prevention was modelled through LDL-C reduction, and the estimates we used suggest the dose of evolocumab considered is slightly more effective at lowering LDL-C than that of alirocumab, with no overlap in the confidence intervals. Because our model was not designed to differentiate between the two in great detail, results should be seen as an indication as to the modelled cost-effectiveness of PCSK9 inhibitors as a class of drug, rather than a recommendation of one over the other.
Cost-effectiveness analyses should be specific to the setting in which treatment is being considered. The structure, organization, and funding of the health system in question should be accounted for in order for results to be meaningful and accurate. Only a limited number of cost-effectiveness analyses have been performed on PCSK9 inhibitors to date, and most were performed in a US setting. Our report offers new insight and perspective in this regard, as Norway’s health system is more similar in nature to other countries in Western and Northern Europe than is the US system.
The most recent analysis from the US found the drugs not to be cost-effective for any patient group.4,14 Researchers considered HeFH patients for both primary and secondary prevention, and general high-risk patients in secondary prevention, which includes those who are statin intolerant. It was estimated that in order for PCSK9 inhibitors to be considered cost-effective for any patient groups, the annual price would need to be reduced by roughly two-thirds. Though, as stated, analyses across different health systems are not always directly comparable due to inherent differences between them, these results appear overall to be consistent with our scenario analysis on price.
The UK’s National Institute for Health and Care Excellence (NICE) has issued guidance recommending use of evolocumab in certain high-risk patient groups. Specifically, it is recommended for secondary prevention of CVD for non-familial hypercholesterolaemia patients at high-risk or very high-risk. It also recommends evolocumab for familial hypercholesterolaemia patients in both primary and secondary prevention settings when LDL-C levels remain very high. The NICE report presents the same general trends as our analyses: a general inverse relationship between risk and ICERs, including lower ICERs with higher LDL-C levels and with higher age. Note that NICE results are based on undisclosed price discounts negotiated with the pharmaceutical company.15
A US-based analysis of evolocumab performed by researchers for Amgen found the drug to be cost-effective in both primary and secondary prevention for familial hypercholesterolaemia patients, and cost-effective in secondary prevention for non-familial hypercholesterolaemia patients, regardless of tolerance to statins. Like our analyses, the Amgen analysis also utilized CTT figures to estimate the effects of LDL-C reduction on the relative risk of CVD.13 The Amgen analysis did not, however, include ezetimibe as a comparator. Because cost-effectiveness modelling focuses on incremental costs and effects between treatment options, the exclusion of ezetimibe is the most likely reason for their differing results.
An analysis presented at an ISPOR conference found that PCSK9 inhibitors as an adjunct to statin therapy were not cost-effective for any patient group tested, regardless of risk or history of CVD. CTT figures were used to estimate risk reductions as a result of LDL-C lowering in this US-based analysis as well.12
Our analyses are also consistent with other analyses in that it finds ezetimibe to be a cost-effective treatment option—and at least one report has found ezetimibe is cost-effective for use specifically in Norway.31,32
Limitations
The lack of data on the actual preventative effect of PCSK9 inhibitors on CVD necessitated that we make a number of assumptions. All CVD prevention was modelled strictly through reductions in LDL-C, which required extrapolation and assumptions based on CTT meta-analyses of statins. This assumes that CVD prevention is strictly a function of LDL-C, and that reductions in LDL-C as a result from PCSK9 inhibition will have the same level of effect as reduction of LDL-C through statins. All of these are major limitations of the analysis, and as such we stress that this is a modelling exercise in potential cost-effectiveness of PCSK9 treatment, rather than a true evaluation of it. Only clinical evidence of CVD prevention as a direct result of using PCSK9 inhibitors can truly address these limitations.
Long-term trials for PCSK9 are currently underway, with expected publication in 2017. In the meantime the method employed in this report, though limited, is thought to be one of the best available options.5 Also, later clinical studies of ezetimibe and rosuvastatin have been shown to reduce LDL-C at levels consistent with CTT estimates, which gives credibility and validity to the extrapolation of CTT beyond their original analyses.8,33 As noted above, other cost-effectiveness analyses also make similar estimations.
Secondary prevention in this analysis is restricted to post-MI patients only, so results exclude stroke patients. Post-IS patients would likely need to be modelled separately, given that they are qualitatively different and have poorer prognoses than post-MI patients.
We did not include the potential for a legacy effect from PCSK9 inhibitors in our analysis, whereby benefits of treatment continue after treatment period has ended. If PCSK9 inhibitors were proven to have a legacy effect, likelihood of cost-effectiveness would presumably rise. A potential legacy effect would be particularly important in younger patients, as it would significantly reduce the long-term costs of treatment.
In addition, our analysis does not account for any ‘saw tooth’ effect on LDL-C, whereby it rises and falls in the intervals between biweekly injections. The potential importance of this factor on long-term CVD risk is currently unknown.
The effects of LDL-C reduction on relative risk of CVD used in this analysis do not differ according to age. CTT does not break down data by both heterogeneity and specific type of CVD prevented; we chose to use the latter as it was more prudent to our analyses.
Some of the analyses performed and results reported are strictly deterministic, when in fact the uncertainty around any analysis should be explored through PSA.34 Specifically, not all of the age groups were tested with PSA, only the 65 and 50 year olds. Results were, however similar in deterministic and probabilistic analyses. The reason it was not undertaken for all analyses is because PSA is computationally demanding and time consuming. Inclusion of more PSA would have lead to a greater exploration of the uncertainty surrounding the results and a more robust analysis.
Conclusion
In conclusion, our model suggests PCSK9 inhibitors would not be cost-effective for primary prevention. In secondary prevention, PCSK9 inhibitors may be cost-effective for older patients at the highest levels of absolute CVD risk. Our model suggests high lifetime drug costs may not be offset by clinical value gained when treating younger patients over many years, regardless of baseline risk. A decrease in price would mean the drugs were more likely to be cost-effective for younger high-risk patients.
Future research is needed to determine the actual long-term preventative effect of PCSK9 inhibitors on CVD.
Supplementary material
Supplementary material is available at European Heart Journal – Cardiovascular Pharmacotherapy online
Conflict of interest: Torbjørn Wisløff has received funding by Amgen in a different project relating to evolocumab.
Supplementary Material
References
- 1. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R.. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stone NJ, Robinson JG, Lichtenstein AH, Merz CNB, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Sanford Schwartz J, Shero ST, Smith SC, Watson K, Wilson PWF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–2934. [DOI] [PubMed] [Google Scholar]
- 3. Reiner Ž, Catapano ALD, Backer G, Graham I, Taskinen M-R, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Filardi PP, Riccardi G, Storey RF, Wood D. ESC/EAS Guidelines for the management of dyslipidaemias. Eur Heart J 2011;32:1769–1818. [DOI] [PubMed] [Google Scholar]
- 4. Tice JA, Ollendorf DA, Cunningham C, Pearson SD.. PCSK9 Inhibitors for Treatment of High Cholesterol: Effectiveness, Value, and Value-Based Price Benchmarks. Institute for Clinical and Economic Review; 2015. [Google Scholar]
- 5. Weintraub WS, Gidding SS.. PCSK9 inhibitors: a technology worth paying for? PharmacoEconomics 2016;34:217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shimada YJ, Cannon CP.. PCSK9 (Proprotein convertase subtilisin/kexin type 9) inhibitors: past, present, and the future. Eur Heart J 2015;36:2415–2424. [DOI] [PubMed] [Google Scholar]
- 7. Fitchett DH, Hegele RA, Verma S.. Statin intolerance. Circulation 2015;131:e389–e391. [DOI] [PubMed] [Google Scholar]
- 8. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW Ferrari GMD, Ruzyllo W, Lucca PD, Im KA, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 9. Pandor A, Ara R, Tumur I, Wilkinson A, Paisley S, Duenas A, Durrington P, Chilcott J.. Ezetimibe monotherapy for cholesterol lowering in 2,722 people: systematic review and meta-analysis of randomized controlled trials. J Intern Med 2009;265:568–580. [DOI] [PubMed] [Google Scholar]
- 10. Lipinski MJ, Benedetto U, Escarcega RO, Biondi-Zoccai G, Lhermusier T, Baker NC, Torguson R, Brewer HB, Waksman R.. The impact of proprotein convertase subtilisin-kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta-analysis. Eur Heart J 2016;37:536–545. [DOI] [PubMed] [Google Scholar]
- 11. Navarese EP, Kołodziejczak M, Schulze V, Gurbel PA, Tantry U, Lin Y, Brockmeyer M, Kandzari DE, Kubica JM, D'Agostino Sr. RB, Kubica J, Volpe M, Agewall S, Kereiakes DJ, Kelm M.. Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia: a systematic review and meta-analysis. Ann Intern Med 2015;163:40–51. [DOI] [PubMed] [Google Scholar]
- 12. Lee H, Danielson D, Watkins J.. Cost-Effectiveness of Intensive Lowering of LDL Cholesterol with PCSK9 Inhibitors as an Adjunct to High-Intensity Statin Therapy for Prevention of Major Vascular Events Poster presented at the International Society of Pharmacoeconomics and Outcomes Research International Meeting, May 21–25, 2016, Washington, DC. 2016.
- 13. Gandra SR, Villa G, Fonarow GC, Lothgren M, Lindgren P, Somaratne R, van Hout B.. Cost-effectiveness of LDL-C lowering with evolocumab in patients with high cardiovascular risk in the United States: cost-effectiveness of evolocumab in the US payer context. Clin Cardiol 2016;39:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kazi DS, Moran AE, Coxson PG, Penko J, Ollendorf DA, Pearson SD, Tice JA, Guzman D, Bibbins-Domingo K.. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA 2016;316:743.. [DOI] [PubMed] [Google Scholar]
- 15. Final Appraisal Determination—Evolocumab for Treating Primary Hypercholesterolaemia and Mixed Dyslipidaemia. National Institute for Health and Care Excellence; 2016. p.80.
- 16. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes J. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet 2005;366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 17. Briggs A, Claxton K, Sculpher M.. Decision Modeling for Health Economic Evaluation. New York: Oxford University Press; 2006. [Google Scholar]
- 18. Wisløff T, Selmer R, Halvorsen S, Kristiansen I.. Norwegian Cardiovascular Disease Model (NorCAD)-a simulation model for estimating health benefits and cost consequences of cardiovascular interventions. Nas Kunnskapssenter Helsetjenesten Oslo nor nor Knowl Cent Health Serv 2008. [Google Scholar]
- 19.Ja til 28 legemidler—nei til 6. Dagens Med. http://www.dagensmedisin.no/artikler/2016/04/25/ja-til-28-legemidler—nei-til-6/ (10 April 2017).
- 20. Augestad MLA, Rand-Hendriksen K, Kristiansen IS, Stavem K.. Impact of transformation of negative values and regression models on differences between the UK and US EQ-5D time trade-off value sets. PharmacoEconomics 2012;30:1203–1214. [DOI] [PubMed] [Google Scholar]
- 21. Dorenkamp M, Bonaventura K, Leber AW, Boldt J, Sohns C, Boldt L-H, Haverkamp W, Frei U, Roser M.. Potential lifetime cost-effectiveness of catheter-based renal sympathetic denervation in patients with resistant hypertension. Eur Heart J 2013;34:451–461. [DOI] [PubMed] [Google Scholar]
- 22. Enden T, Resch S, White C, Wik H, Kløw N, Sandset P.. Cost-effectiveness of additional catheter-directed thrombolysis for deep vein thrombosis. J Thromb Haemost 2013;11:1032–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wisløff T. Cost-effectiveness of antiplatelet drugs after percutaneous coronary intervention. Eur Heart J Qual Care Clin Outcomes 2016;2:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wisløff T, Atar D, Kristiansen IS.. Cost effectiveness of drug-eluting stents as compared with bare metal stents in patients with coronary artery disease. Am J Ther 2013;20:596–601. [DOI] [PubMed] [Google Scholar]
- 25. Wisløff T, Hagen G, Klemp M.. Economic evaluation of warfarin, dabigatran, rivaroxaban, and apixaban for stroke prevention in atrial fibrillation. PharmacoEconomics 2014;32:601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Innsatsstyrt finansiering 2016 [Activity-based funding in 2016]. Helsedirektoratet; 2015. Report No.: IS-2417. ISBN: 978-82-8081-417-3.
- 27. Legemiddelverk (Norwegian Medicines Agency). Legemiddelverket. http://www.legemiddelverket.no/Sider/default.aspx (10 April 2017).
- 28. Normaltariff for fastleger og legevakt 2015-2016 [Normal Tariff for GPs and emergency care 2015-2016]. Den norske legeforening; 2015.
- 29. Norwegian Prescription Database. http://www.norpd.no/ (10 April 2017).
- 30. Norheim OF, Gjelsvik B, Klemsdal TO, Madsen S, Meland E, Narvesen S, Negard A, Njolstad I, Tonstad S, Ulvin F, Wisloff T.. Norway’s new principles for primary prevention of cardiovascular disease: age differentiated risk thresholds. BMJ 2011;343:d3626–d3626. [Google Scholar]
- 31. Cook JR, Yin D, Alemao E, Davies G, Krobot KJ, Veltri E, Lipka L, Badia X.. Cost-effectiveness of ezetimibe coadministration in statin-treated patients not at cholesterol goal. Pharmacoeconomics 2004;22:49–61.. [DOI] [PubMed] [Google Scholar]
- 32. Ara MR, Pandor A, Tumur I, Paisley S, Duenas A, Williams R, Rees A, Wilkinson A, Durrington P, Chilcott J.. Cost Effectiveness of Ezetimibe in Patients with Cardiovascular Disease and Statin Intolerance or Contraindications. Am J Cardiovasc Drugs 2008;8:419–427. [DOI] [PubMed] [Google Scholar]
- 33. Claxton K, Sculpher M, McCabe C, Briggs A, Akehurst R, Buxton M, Brazier J, O’hagan T.. Probabilistic sensitivity analysis for NICE technology assessment: not an optional extra. Health Econ 2005;14:339–347. [DOI] [PubMed] [Google Scholar]
- 34. Yusuf S, Bosch J, Dagenais G, Zhu J, Xavier D, Liu L, Pais P, López-Jaramillo P, Leiter LA, Dans A, Avezum A, Piegas LS, Parkhomenko A, Keltai K, Keltai M, Sliwa K, Peters RJG, Held C, Chazova I, Yusoff K, Lewis BS, Jansky P, Khunti K, Toff WD, Reid CM, Varigos J, Sanchez-Vallejo G, McKelvie R, Pogue J, Jung H. et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016;374:2021–2031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.