Abstract
Aim
Long-term prognostic impact of coronary artery disease (CAD) severity in stable post-myocardial infarction (MI) patients is not well known. We examined the impact of CAD severity and co-morbidity on the long-term (1 year and beyond) risk of cardiovascular events post-MI.
Methods and results
From nationwide administrative and clinical registers, we identified 55 747 MI patients, during 2004–2010, who had not experienced subsequent MI, stroke, or death within 7 days post-discharge. The risk for primary composite endpoint (MI, stroke, or cardiovascular death) was estimated for the first 365 days after MI (index MI) and from day 366 to study completion (stable post-MI population), corresponding to a mean follow-up of 3.6 (2.2) years. Risk was assessed using cumulative incidence, multivariable adjusted logistic regression and Cox proportional-hazards models. The 1-year cumulative incidence for primary endpoint was 20.0% [95% confidence interval (CI), (19.6–20.3)]. Correspondingly, the 4-year cumulative incidence for primary endpoint was 21.0% (95% CI, 20.6–21.4) in patients without events on the first year. In multivariable models with no significant stenosis as reference, CAD severity was the most important risk factor for cardiovascular events the first 365 days [left main stenosis (LMS): odds ratio and 95% CI, 4.37, 3.69–5.17; 3-vessel disease (VD), 4.18, 3.66–4.77; 2-VD, 3.23, 2.81–3.72; 1-VD, 2.12,–1.85–2.43] and remained from day 366 to study completion [LMS: hazard ratio and 95% CI, 1.91, 1.64–2.22; 3-VD, 1.85,1.65–2.07; 2-VD, 1.55, 1.38–1.74; 1-VD, 1.30, 1.16–1.45].
Conclusion
Despite contemporary treatment at baseline, stable post-MI patients’ 4-year outcome was similar to 1-year outcome after MI, and CAD severity remained a critical risk factor the first year and thereafter.
Keywords: Coronary artery disease, Myocardial infarction, Multi-vessel disease, Cardiovascular risk factors
Introduction
Improved lifestyle, together with more effective pharmacotherapy and invasive treatment, has resulted in a decline in first-time coronary artery disease (CAD) and increased survival in patients with established CAD.1,2 Despite the decline in mortality from CAD, it remains one of the leading causes of premature death on a European scale.2 Aside from an additional risk of premature death, CAD is also associated with risk of recurrent cardiovascular events, e.g. stroke and recurrent myocardial infarction (MI), with the highest risk of recurrent events during the first year after MI.3–5 This risk is targeted by a similar guideline recommended treatment duration, but evidence has shown that the risk persists beyond the first year after MI and that the risk depends on the patient’s risk profile.4–6 With the projected increase in high-risk patients that stay event-free on the first year after MI and the associated long-term health-care burden,6 it has become even more relevant to clarify the long-term risk of recurrent events in stable post-MI patients with distinct risk profiles. Thus, high-risk patients might benefit from extended tailored treatment approach. However, it is important to highlight the fact that a considerable proportion of patients with established illness still appear to receive sub-optimal cardiac care,7 secondary prevention and cardiac rehabilitation.8 Another serious challenge is that the prevalence of coexisting chronic illnesses, which in many cases share the same risk factors as CAD,9 is high and increasing,1 but even more importantly, are associated with unfavourable prognosis in patients with CAD.1,10–14 Although CAD severity is one of the strongest risk factors for long-term outcome,15–20 its importance in late-risk stratification and in relation to co-morbidity among stable post-MI patient who have stayed event-free on the first year is not entirely clear. Recognizing that CAD extent and severity can been graded differently from the more simple usage of the number of stenosed vessels15–17 to the use of more complex scoring system21 and that the coronary angiography (CAG) lack of ability to detect prognostically important physiological stenosis,22 we investigated the long-term (1 year and beyond) impact of CAD severity, using the simple estimation of CAD severity (no obstructive CAD, 1-, 2-, 3-vessel disease [VD] or left main stenosis [LMS]), in relation to co-morbidity for recurrent events in a nationwide study population. Patients conservatively treated who were not receiving CAG, and thus with no information on CAD severity, were also included, as conservatively treated patients fare worse than invasively treated patients and deserve equal attention in terms of attaining knowledge of risk and risk factors.
Methods
Data sources
In Denmark, each resident has a unique and permanent identification number that enables individual-level linkage among several Danish nationwide administrative registries, allowing record linkage analysis. (i) The Civil Registrations System holds information on sex, year of birth, civil status and the unique identifier on each Danish resident since 1968.23 (ii) The Danish National Patient Registry (DNPR) holds information on dates of admission and discharge, main and secondary discharge diagnoses according to the International Classification of Diseases, 10th revision (ICD-10), from 1994, and surgical procedure codes according to the NOMESCO Classification of Surgical Procedures (NCSP) from 1996. Since 2002, the DNPR has used the Diagnosis-Related Group system for hospital reimbursement.24 (iii) The Danish Register of Causes of Death keeps records on date and cause(s) of death classified according to ICD-10 since 1994.25 (iv) The Danish Heart Registry (DHR) is a clinical quality database that keeps track on invasive examinations and treatments, which include CAG, percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) since 2000. In addition, risk factors such as diabetes mellitus (DM) are recorded as well. The DHR has national coverage on CAG since 2006. National coverage is defined as more than 89% of the produces recorded in the DHR as well as in the DNPR in relation to the number of procedures registered in the DNPR. Each hospital performing CAG, PCI, or CABG is obligated to report data on performed procedures to the DHR.26 (v) The Danish National Registry of Medicinal Product Statistics holds information on date of dispensing, quantity dispensed, strength and formulation of all partially reimbursed prescription drugs dispensed from Danish pharmacies since 1995. Each drug dispensing is classified according to the International Anatomical Therapeutic Chemical (ATC) system.27 (vi) The Integrated Database for Labour Market Research holds information on taxed income gathered by government tax authorities.
Study population
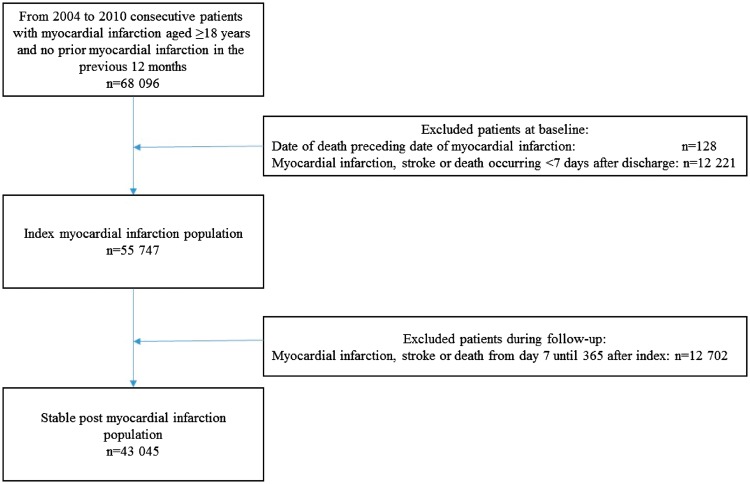
We identified all patients ≥18 years with first recorded primary or secondary diagnosis of index MI (ICD-10: I21) from 1 January 2004 to 31 December 2010, and who had no prior MI admissions registered in the previous 12 months (Figure 1). Patients with a recent MI history were not included, because they probably had a different risk profile per se and were most likely already in dual treatment and revascularized.
Figure 1.
Selection of study populations.
Myocardial infarction population
Patients who survived index MI, without stroke or recurrent MI within 7 days from discharge, were included. The quarantine period from index MI to 7 days after discharge, at which point all were alive, was chosen to allow assessment of medication at discharge. Co-morbidities, comprising prior MI, cerebrovascular disease, peripheral arterial disease, heart failure, arrhythmia, shock, pulmonary oedema, acute renal failure, chronic renal failure, DM, cancer, respiratory insufficiency, chronic obstructive pulmonary disease, anaemia, and infection, were defined as the presence of discharge diagnoses up to 10 years prior to the index MI, but prior MI was identified if any diagnosis since 1994. Identification of important co-morbidities in an unselected MI population was based on the Ontario acute MI mortality prediction rules,28 which was modified by expanding the time window for co-morbidity identification, identifying DM with as well as without complications and specifying additional co-morbidities of importance, including for type 2 MI.29,30 The incomplete capture of discharge code-based heart failure and DM was compensated by including loop diuretics and glucose-lowering drugs. Arrhythmia indicated cardiac arrest, paroxysmal tachycardia, atrial fibrillation/flutter, and other cardiac arrhythmias. Shock indicated cardiogenic, hypovolaemic, or other/unspecified shock. Infection indicated urinary tract infection and sepsis of bacterial or fungal origin. Revascularization was measured up to 7 days after hospital discharge. Concomitant medication was defined as redeemed prescriptions from 365 days before hospital admission until 7 days after discharge. Socioeconomic status was measured by means of 5-year average index income (in quintiles) and civil status (married, living with a partner or living alone).
Stable post-myocardial infarction population
For ‘stable’ post-MI patients, who survived the first 365 days after the index MI, without a stroke or recurrent MI, identification of co-morbidities (discharge- as well as drug-based codes) was extended until 365 days after discharge. Revascularization was measured up to 365 days after hospital discharge. Concomitant medication (excluding loop diuretics and glucose-lowering drugs) was defined as redeemed prescriptions from day 244 to day 365 after discharge. Socioeconomic status was measured at index MI. A full list of the ICD-10, procedure (NCSP) and ATC codes used to identify co-morbidity, revascularization and medication is provided in the Supplementary material online, Table S1.
Coronary artery disease severity
Identification of CAD severity was based on the findings from CAG and PCI performed up to 7 and 365 days after hospital discharge for the index MI and stable post-MI population, respectively. CAD severity was defined according to the number of obstructive coronary arteries corresponding to 50% or more narrowing and categorized into 7 groups: no significant stenosis, 1-, 2-, 3- VD, LMS, missing angiographic data or if no CAG was performed. LMS with or without additional diseased vessels were categorized as LMS only. For patients with >1 angiography record, the record with the most severe disease was retained for the analysis. Multi-vessel CAD (MVD) was defined as 2- or 3-VD or LMS.
Endpoint and follow-up
The primary composite endpoint was defined as the first recorded primary or supplementary diagnosis of MI (ICD10: I21), ischemic stroke (ICD10: I63, I64) or fatal cardiovascular disease (CVD) (ICD10: I00-I99). MI or stroke was considered to be non-fatal regardless of subsequent death at a later point in time (i.e. ≥1 day after non-fatal MI/stroke) and non-fatal MI or stroke appearing at the same time was classified as non-fatal MI. For the index MI patients, the length of follow-up was defined as the time elapsed from day 7 after hospital discharge until the primary composite endpoint, death, emigration, or end of 1-year follow-up (day 365 after discharge). For the stable post-MI patients, the length of follow-up was defined as the time elapsed from day 366 after hospital discharge until the primary composite endpoint, death, emigration, or end of study follow-up (31 December 2012).
Statistical methods
Continuous and categorical variables were presented as median (interquartile range) and as frequencies (%), respectively. Kruskal–Wallis test was used for comparison of continuous variables, whereas chi-square test was used for comparison of categorical variables. Crude incidence rates of the composite endpoint and its components were calculated per 100 person-years (PY). The overall crude incidence rate of the composite endpoint was also stratified according to CAD severity, which was a constructed ordinal variable: no significant stenosis, 1-, 2-, 3-VD, LMS, missing data on CAD severity or if no CAG. The 1-year (from day 7 until day 365 after discharge) and beyond (from day 366 until end of study) cumulative incidence curves for the composite endpoint according to CAD severity were estimated using the Nelson–Aalen estimator that account for the competing event of death from non-cardiovascular causes. Logistic regression and Cox proportional-hazards models were used to estimate the 1-year and beyond impact of CAD severity and co-morbidity on the composite endpoint, respectively. The models were adjusted for potential confounders, which included age, age groups, sex, calendar year, revascularization status, medication and socioeconomic status (income and civil status). No significant stenosis, the age group of 50-59 years, highest yearly income and married as civil status served as reference groups for the analyses. Patients with missing data on CAD severity (around 8%) were excluded in the logistic regression and Cox proportional-hazard models (complete case analyses). Three sensitivity analyses of the 1-year and beyond impact of CAD severity and co-morbidity on composite endpoint were conducted with stepwise exclusion of: (i) those with missing information on CAD; (ii) those with index MI in year 2010 because of disproportionately high occurrence of missing information on CAD severity; and (iii) those who did not receive CAG. The proportional hazard assumption, linearity of continuous variable, and lack of interaction were found to be valid unless otherwise indicated. All statistical analyses and data management were carried out using SAS, version 9.4 (SAS Institute, Cary, NC, USA) and R statistic software (version 3.1.1). P-values less than 0.05 were considered statistically significant.
Ethics
Register-based studies do not require ethical approval according to Danish legislation. Approval was granted by the Danish Data Protection Agency (Ref.no. 2007-58-0015/local ref. GEH-2014-014 I-Suite no: 02732).
Results
Study populations
During the study period, January 2004 to December 2010, 68 096 MI patients aged 18 years or older were hospitalized, of whom 55 747 (81.9%) patients survived and did not experience a recurrent MI or stroke 7 days after hospital discharge and were included in the study. Of the MI population, 43 045 patients (77.2%) survived 365 days without any subsequent MI or stroke and were classified as the stable post-MI population with a mean duration of follow-up time of 3.6 years and maximum follow-up time of 9 years (Figure 1 and Table 1).
Table 1.
Baseline characteristics of the index myocardial infarction population 7 days after hospital discharge and for the stable post-myocardial infarction population 366 days after discharge
| Index MI | Stable post-MI | |
|---|---|---|
| n = 55 747 | n = 43 045 | |
| Age (IQR), years | 70 (20) | 68 (20) |
| Age groups, years | ||
| ≤49 | 4992 (9.0) | 4033 (9.4) |
| 50–59 | 8779 (15.7) | 7362 (17.1) |
| 60–69 | 13 277 (23.8) | 11 096 (25.8) |
| 70–79 | 14 153 (25.4) | 10 656 (24.8) |
| ≥80 | 14 546 (26.1) | 9898 (23.0) |
| Male | 35 609 (63.9) | 28 130 (65.4) |
| CAD severity | ||
| No significant stenosis | 4145 (7.4) | 4050 (9.4) |
| 1-VD | 15 122 (27.1) | 14 011 (32.5) |
| 2-VD | 7777 (14.0) | 6769 (15.7) |
| 3-VD | 7020 (12.6) | 5566 (12.9) |
| LMS | 1607 (2.9) | 1216 (2.8) |
| Missing data on CAD severity | 4223 (7.6) | 3.577 (8.3) |
| No performed CAG | 15 853 (28.4) | 7856 (18.3) |
| Co-morbidity | ||
| Prior MI | 4413 (7.9) | 3043 (7.1) |
| Cerebrovascular disease | 5526 (9.9) | 3541 (8.2) |
| Peripheral arterial disease | 2239 (4.0) | 1739 (4.0) |
| Heart failurea | 19 750 (35.4) | 17 035 (39.6) |
| Arrhythmiab | 10 086 (18.1) | 8846 (20.6) |
| Shockc | 218 (0.4) | 190 (0.4) |
| Pulmonary oedema | 686 (1.2) | 526 (1.2) |
| Acute renal failure | 1073 (1.9) | 830 (.9) |
| Chronic renal failure | 1330 (2.4) | 1040 (2.4) |
| Diabetes mellitusd | 11 290 (20.3) | 9048 (21.0) |
| Cancer | 3852 (6.9) | 2959 (6.9) |
| Respiratory insufficiency | 1203 (2.2) | 980 (2.3) |
| Chronic obstructive pulmonary disease | 4861 (8.7) | 3803 (8.8) |
| Anaemia | 3317 (6.0) | 2955 (6.9) |
| Infectione | 1245 (2.2) | 1087 (2.5) |
| Revascularization | ||
| PCI | 25 425 (45.6) | 24 050 (55.9) |
| CABG | 2331 (4.2) | 3644 (8.5) |
| No revascularization | 30 322 (54.4) | 16 201 (37.6) |
| Concomitant medicationf | ||
| β-Blockers | 41 712 (74.8) | 30 602 (71.1) |
| Lipid-lowering treatment | 41 971 (75.3) | 32 639 (75.8) |
| Aspirin | 46 540 (83.5) | 34 235 (79.5) |
| Nitrate | 16 030 (28.8) | 7489 (17.4) |
| P2Y12 inhibitors | 34 882 (62.6) | 26 144 (60.7) |
| Clopidogrel | 34 802 (62.4) | 26 037 (60.5) |
| Ticagrelor | 0 | 4 (0.1) |
| Prasugrel | 97 (0.2) | 106 (0.3) |
| Glucose-lowering drugs | 7528 (13.5) | 5901 (13.7) |
| Loop diuretics | 17 373 (31.2) | 14 185 (33.0) |
| Vitamin-K antagonist/NOAC | 4277 (7.7) | 2759 (6.4) |
| Spironolactone | 4562 (8.2) | 3248 (7.5) |
| NSAID | 16 207 (29.1) | 5016 (11.7) |
| PPI | 15 344 (27.5) | 9580 (22.3) |
| Socioeconomic factors | ||
| Yearly family income in quintiles | ||
| 1 | 10 680 (19.2) | 7179 (16.7) |
| 2 | 10 538 (18.9) | 7322 (17.0) |
| 3 | 10 962 (19.7) | 8317 (19.3) |
| 4 | 11 626 (20.9) | 9688 (22.5) |
| 5 (highest) | 11 941 (21.4) | 10 539 (24.5) |
| Civil status | ||
| Married | 29 553 (53.0) | 23 968 (55.7) |
| Living with a partner | 3362 (6.0) | 2864 (6.7) |
| Living alone | 22 832 (41.0) | 16 213 (37.7) |
Continuous and categorical variables were expressed as median (IQR) and frequency (%), respectively.
MI, myocardial infarction; IQR, interquartile range; CAD, coronary artery disease; VD, vessel disease; LMS, left main stenosis; CAG, coronary angiography; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; P2Y12, antiplatelet inhibitors; NOAC, new oral anticoagulants; NSAID, non-steroid anti-inflammatory drug; PPI, proton pump inhibitor.
Heart failure was defined as any discharge code indicative of heart failure or use of loop diuretics.
Arrhythmia was defined as any discharge code indicative of cardiac arrest, paroxysmal tachycardia, atrial fibrillation/flutter, and other cardiac arrhythmias.
Shock was defined as any discharge code indicative of cardiogenic, hypovolaemic, or other/unspecified shock.
Diabetes mellitus was defined as any discharge code indicative of diabetes mellitus or use of glucose-lowering drugs.
Infection was defined as any discharge code indicative of urinary tract infection and sepsis of bacterial or fungal origin.
Redeemed prescriptions of concomitant medication: from 365 days before until 7 days after discharge for the index MI population, from day 244 to day 365 after discharge for the post-MI population, except for loop diuretics and glucose-lowering drugs, which for the stable group covered the period from 365 before until 365 days after discharge.
Irrespective of study population, when stratified according to CAD severity, age, and co-morbidity burden increased with increasing CAD severity, but at the same time, the proportion of revascularized and treatment with P2Y12 inhibitors declined as opposed to nitrates. Nevertheless, patients who did not receive CAG were older, had a greater co-morbidity burden, and received less optimal medication (Tables 2 and 3, Supplementary material online, Tables S2 and S3).
Table 2.
Index myocardial infarction patients’ treatment regimen by coronary artery disease severity
| No significant stenosis n = 4145 | 1-VD | 2-VD | 3-VD | LMS | Missing data on CAD severity n = 4223 | No CAG n = 15 853 | P-value | |
|---|---|---|---|---|---|---|---|---|
| n = 15 122 | n = 7777 | n = 7020 | n = 1607 | |||||
| Revascularization | ||||||||
| PCI | 131 (3.2) | 13 094 (86.6) | 6110 (78.6) | 3407 (48.5) | 649 (40.4) | 2034 (48.2) | 0 (0) | <0.001 |
| CABG | 16 (0.4) | 107 (0.7) | 302 (3.9) | 1189 (16.9) | 393 (24.5) | 324 (7.7) | 0 (0) | <0.001 |
| No revascularization | 3998 (96.5) | 1969 (13.0) | 1434 (18.4) | 2574 (36.7) | 623 (38.8) | 1884 (44.6) | 15 853 (100) | <0.001 |
| Concomitant medicationa | ||||||||
| Nitrate | 873 (21.1) | 3075 (20.3) | 2128 (27.4) | 2676 (38.1) | 648 (40.3) | 1101 (26.1) | 5529 (34.9) | <0.001 |
| β-Blockers | 2717 (65.5) | 12 926 (85.5) | 6423 (82.6) | 5281 (75.2) | 1162 (72.3) | 3271 (77.5) | 9932 (62.7) | <0.001 |
| Lipid-lowering treatment | 2963 (71.5) | 13 761 (91.0) | 6910 (88.9) | 5743 (81.8) | 1254 (78.0) | 3552 (84.1) | 7788 (49.1) | <0.001 |
| Aspirin | 3026 (73.0) | 13 604 (90.0) | 6818 (87.7) | 5724 (81.5) | 1290 (80.3) | 3646 (86.3) | 12,32 (78.4) | <0.001 |
| P2Y12 inhibitors | 1918 (46.3) | 12 821 (84.8) | 6161 (79.2) | 4364 (62.2) | 930 (57.9) | 2961 (70.1) | 5727 (36.1) | <0.001 |
Categorical variables expressed as frequency (%). VD, vessel disease; LMS, left main stenosis; CAD, coronary artery disease; CAG, coronary angiography; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; P2Y12, antiplatelet inhibitors. aRedeemed prescriptions of concomitant medication: from 365 days before until 7 days after discharge. Differences between the groups were found using chi-square test.
Table 3.
Stable post-myocardial infarction patients’ treatment regimen by coronary artery disease severity
| No significant stenosis n = 4050 | 1-VD | 2-VD | 3-VD | LMS | Missing data on CAD severity n = 3577 | No CAG n = 7856 | P-value | |
|---|---|---|---|---|---|---|---|---|
| n = 14 011 | n = 6769 | n = 5566 | n = 1216 | |||||
| Revascularization | ||||||||
| PCI | 234 (5.8) | 12 532 (89.4) | 5715 (84.4) | 3102 (55.7) | 571 (47.0) | 1896 (53.0) | 0 (0) | <0.001 |
| CABG | 27 (0.7) | 189 (1.3) | 559 (8.3) | 1850 (33.2) | 534 (43.9) | 485 (13.6) | 0 (0) | <0.001 |
| No revascularization | 3790 (93.6) | 1387 (9.9) | 687 (10.1) | 1007 (18.1) | 244 (20.1) | 1230 (34.4) | 7856 (100) | <0.001 |
| Concomitant medicationa | ||||||||
| Nitrate | 560 (13.8) | 1786 (12.7) | 1118 (16.5) | 1242 (22.3) | 267 (22.0) | 533 (14.9) | 1983 (25.2) | <0.001 |
| β-Blockers | 2288 (56.5) | 10 900 (77.8) | 5230 (77.3) | 4362 (78.4) | 923 (75.9) | 2641 (73.8) | 4258 (54.2) | <0.001 |
| Lipid-lowering treatment | 2597 (64.1) | 11 944 (85.2) | 5777 (85.3) | 4831 (86.8) | 1056 (86.8) | 2941 (82.2) | 3493 (44.5) | <0.001 |
| Aspirin | 2702 (66.7) | 11 931 (85.2) | 5713 (84.4) | 4581 (82.3) | 1012 (83.2) | 2939 (82.2) | 5357 (68.2) | <0.001 |
| P2Y12 inhibitors | 1356 (33.5) | 11 057 (78.9) | 5202 (76.9) | 3540 (63.6) | 756 (62.2) | 2259 (63.2) | 1974 (25.1) | <0.001 |
Categorical variables expressed as frequency (%).
VD, vessel disease; LMS, left main stenosis; CAD, coronary artery disease; CAG, coronary angiography; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; P2Y12, antiplatelet inhibitors.
Redeemed prescriptions of concomitant medication from 244 to 365 days after discharge; B, from day 244 to day 365 after discharge. Differences between the groups were found using chi-square test.
Furthermore, utilization of CAG, revascularization, and P2Y12 inhibitors increased as opposed to nitrates over the course of time. Patients enrolled in the early period were older and had more prevalent cardiac co-morbidities (MI and heart failure) (see Supplementary material online, Tables S4 and S5).
Myocardial infarction population
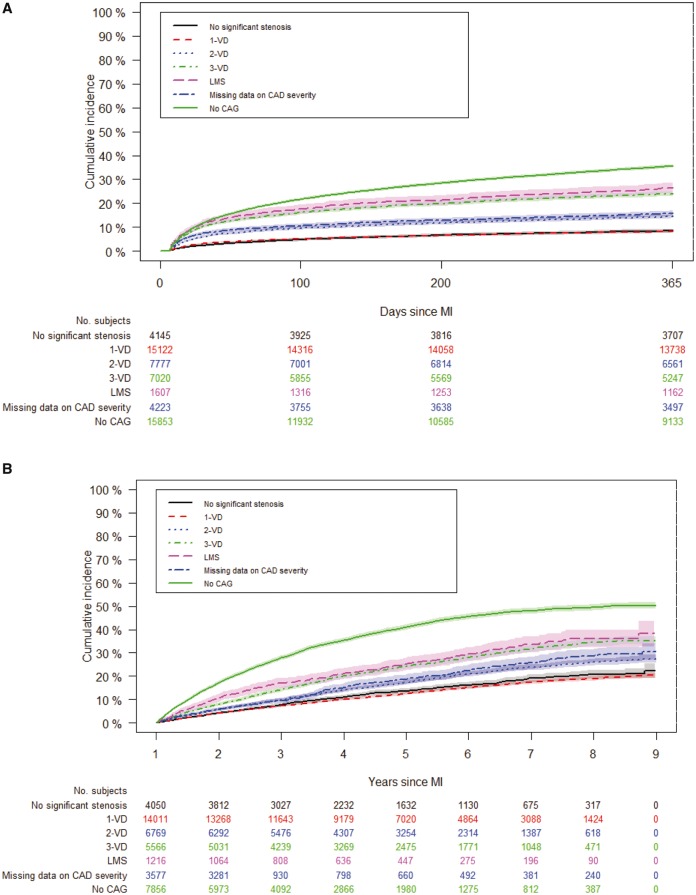
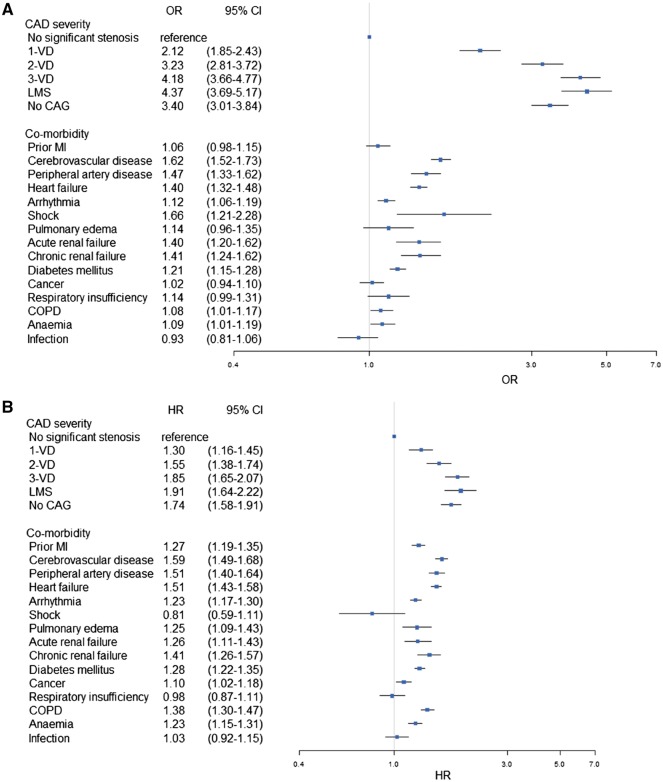
Around 72% of the MI patients underwent CAG of which 41.1% had MVD. A total of 20 467 primary composite endpoint events (non-fatal MI, 46.0%; non-fatal stroke, 13.6%; cardiovascular death, 40.5%) were observed [10.2/100 PYs; 95% confidence interval (CI), 10.1-10.3], of which 11 129 events (non-fatal MI, 51.9%; non-fatal stroke, 10.0%; cardiovascular death, 38.2%) occurred within the first 365 days (23.8/100 PYs; 95% CI, 23.4–24.3), which corresponded to 20.0% (19.6–20.3), when accounting for the competing risk of non-cardiovascular death (Table 4 and Supplementary material online, Table S6). The incidence rate of the primary composite endpoint increased with increasing CAD severity but was highest in patients who did not receive CAG. Similarly, the cumulative risk of primary composite endpoint the first 365 days post-index MI rose from 8.4% (7.6–9.2) in those with no significant stenosis to 26.4% (24.3–28.6) in those with LMS (Figure 2A and Table 4). A higher cumulative risk was noted in patients with unknown CAD severity due to lacking invasive examination (35.5%, 34.8–36.3). After controlling for confounders, CAD severity was the most important risk factor in relation to co-morbidity (Figure 3A). Other important risk factors were increasing age, male gender, cerebrovascular disease, peripheral artery disease, shock, not receiving revascularization, not receiving secondary preventive medication (β-blockers, statin, aspirin, and P2Y12 inhibitors) and use of nitrates (Supplementary material online, Figure S1).
Table 4.
Incidence rate (per 100 person-years) and cumulative incidence with 95% confidence interval of the composite endpoint according to time since index myocardial infarction and after becoming stable post-myocardial infarction
| Index MI population |
Stable post-MI population |
|||
|---|---|---|---|---|
|
n = 55 747 |
n = 43 045 |
|||
| Incidence rates | Cumulative incidence | Incidence rates | Cumulative incidenceb | |
| Composite endpointa | 23.8 (23.4–24.3) | 20.0 (19.6–20.3) | 6.1 (5.9–6.2) | 21.0 (20.6–21.4) |
| No significant stenosis | 9.0 (8.1–10.0) | 8.4 (7.6–9.2) | 3.8 (3.5–4.1) | 13.6 (12.5–14.8) |
| 1-VD | 8.7 (8.2–9.1) | 8.2 (7.8–8.7) | 3.4 (3.2–3.5) | 12.6 (12.0–13.2) |
| 2-VD | 16.4 (15.5–17.4) | 14.5 (13.8–15.3) | 4.8 (4.6–5.1) | 17.3 (16.3–18.3) |
| 3-VD | 29.5 (28.1–30.9) | 24.0 (23.0–25.0) | 6.8 (6.5–7.2) | 24.3 (23.1–25.5) |
| LMS | 33.2 (30.2–36.5) | 26.4 (24.3–28.6) | 7.5 (6.7–8.4) | 25.2 (22.5–27.8) |
| Missing data on CAD severity | 29.4 (26.8–32.3) | 15.9 (14.7–17.0) | 7.6 (6.8–8.4) | 18.5 (16.5–20.5) |
| No CAG | 50.4 (49.1–51.8) | 35.5 (34.8–36.3) | 13.3 (12.9–13.8) | 41.0 (39.8–42.1) |
MI, myocardial infarction; VD, vessel disease; LMS, left main stenosis; CAD, coronary artery disease; CAG, coronary angiography. aNon-fatal MI, non-fatal stroke or cardiovascular death.
Four years after becoming stable.
Figure 2.
Cumulative incidence of composite endpoint in the index myocardial infarction (A) and stable post myocardial infarction population (B) according to coronary artery disease severity. A: day 7 after discharge until 1-year follow-up and B: day 366 after discharge until end of follow-up. VD, vessel disease; CAD, coronary artery disease; CAG, coronary angiography.
Figure 3.
Multivariable analyses showing the impact of CAD severity and co-morbidity on composite endpoint event in the index myocardial infarction population (A) and stable post-myocardial infarction population (B). The multivariable analysis is based on a complete case approach and adjusted for age, age-groups, gender, calendar year, revascularization, pharmacotherapy, and socioeconomic status. OR, odds ratio; HR, hazard ratio; CI, confidence interval; CAD, coronary artery disease; VD, vessel disease; CAG, coronary angiography; LMS, left main stenosis; MI, myocardial infarction; COPD, chronic obstructive pulmonary disease.
Stable post-myocardial infarction population
Among the almost 82% of the stable post-MI patients that received CAG, 38.5% had MVD. Until study completion 9338 (non-fatal MI, 38.9%; non-fatal stroke, 17.9%; cardiovascular death, 43.2%) experienced a composite endpoint event (6.1/100 PYs; 95% CI, 5.9–6.2) (Table 4 and Supplementary material online, Table S6). A similar trend was noted here. The incidence rate of the primary composite endpoint increased with increasing CAD severity, but was highest in patients who did not receive CAG. Similarly, the cumulative incidence of the composite endpoints at 4 years follow-up after becoming stable was overall 21.0% (20.6–21.4), but 13.6% (12.5–14.8) in patients with no significant stenosis and 25.2% (22.5–27.8) in patients with LMS (Table 4 and Figure 2B). For patients who did not receive invasive examination, the risk was higher (41.0%, 39.8–42.1). After adjusting for confounders, CAD severity remained as the most important risk factor, but its relative importance in relation to co-morbidity was less pronounced (Figure 3B). Additionally, important risk factors were increasing age, male gender, cerebrovascular disease, peripheral artery disease, heart failure, not receiving revascularization, not receiving statins and use of nitrates (Supplementary material online, Figure S2).
Sensitivity analysis
To test the robustness of our results, stepwise sensitivity analyses were carried out for both study populations. Excluding patients with missing information showed no changes for the risk of events. Data from the DHR in 2010 showed a higher proportion of missing data than in the preceding years (see Supplementary material online, Tables S4 and S5), and by restricting the analysis to the 2004–2009 period no appreciable changes were noted. By further restricting the analyses to patients with known CAD severity status only, the CAD severity-stratified estimates remained identical, but the overall estimate reduced from 20.0% (19.6–20.3) to 13.50% (13.1–13.9) in the index population. Correspondingly, the overall estimate in the stable post-MI population changed from 21.0% (20.6–21.4) to 16.3% (15.9–16.8) (data not shown). A similar stepwise approach was carried out for the multivariable analyses and no appreciable changes were noted for co-morbidity impact on outcome (see Supplementary material online, Tables S7 and S8), but for CAD severity, an increase in the estimates was noted but with overlapping CIs.
Discussion
In this nationwide cohort study, encompassing almost 56 000 MI patients with a follow-up period of up to 9 years, we examined the risk of recurrent cardiovascular events (non-fatal MI, non-fatal stroke or cardiovascular death) in 2 distinct study populations. The key findings were: (i) one in five patients experienced a recurrent cardiovascular event the first 365 days, but for patients surviving the first 365 days without a recurrent cardiovascular event after MI, the risk remained equally high, with one in five patients experiencing an event later; (ii) CAD severity remained as the most critical factor for recurrent cadiovascular events both before and after the first 365 days; (iii) co-morbidity was a strong risk factor for cardiovascular events, but its relative importance was more pronounced in the long term.
The findings from this present study add valuable knowledge to the limited evidence on impact of CAD severity on recurrent events in a nationwide post-MI cohort who had been stable for at least 1 year. Using CAD severity, which is a well-established risk factor for cardiovascular events to identify high-risk patients, we found a dose–response relationship, but since CAD severity had been modified by important factors such as revascularization and pharmacotherapy at baseline, which differed across CAD severity, we did not measure the true effect of CAD severity. Furthermore, patients with no significant stenosis and to a certain extent those conservatively treated probably had a different underlying cause for MI,22,31 but examining the extremes of MI patients is valuable, particularly because these patients are not rare and pose a clinical challenge.
Prevalence of multi-vessel disease
In this present nationwide study on MI with a small percentage of non-naïve patients, the prevalence of MVD (2-VD or higher) among CAG recipients was about 41%. This is consistent with a previous Danish study on first-time MI.32 Another study, with an almost similar setup to our study, reported a nearly identical prevalence of MVD (38%) in the index MI,4 and as expected, a lower prevalence of 30% was reported when only focusing on 3-VD, LMS, and prior CABG.3 The ICD-10 classification system does not account for the different subtypes of MI. Current evidence suggests that type 1 MI [traditionally corresponding to ST-segment elevation (STEMI) and non-ST-segment elevation (NSTEMI)] constitutes the largest group.33 In STEMI and NSTEMI studies, MVD counts for around 50%.34–36 Although almost 39% had MVD in the stable post-MI population in the present study, others have reported in the range of 34–58%,37–39 reflecting important differences in how their stable populations were selected. Altogether, the prevalence of MVD in MI and stable post-MI is comparable with national and international findings, but more importantly, this shows that a large proportion of MI patients surviving the first year without events had a high coronary atherosclerotic burden.
Furthermore, we showed in both populations that age and co-morbidity burden increased with increasing CAD severity, which is in line with other studies.3,39–41 As expected, we found that MI patients being event-free on the first year had a more favourable risk profile (younger, lower proportion of MVD and less degree of prior MI and stroke), an observation also made by others.5 On that note, a considerable proportion of MI did not receive CAG. These patients were older and most likely had a higher occurrence of MVD, which in turn means that the true number of MVD was probably underestimated. Nevertheless, the complexity of MVD patients underscores the need for a coordinated effort involving optimal treatment of CAD along with appropriate management of significant co-morbidities. The importance of this is emphasized further by the expected increasing prevalence of CAD combined with the high and increasing burden of co-morbidity,1 leading ultimately to an increasing economic burden on the health-care system.
Cardiovascular risk and risk factors
We demonstrated a considerable residual risk for cardiovascular events during the first year and the first 4 years of follow-up after becoming stable post-MI. More precisely, one out of 5 MI survivors experienced an event the first year and the same risk was observed after 4 years in stable post-MI patients. Studies on long-term prognosis have used different approach to identify high-risk patients.4–6 Similar to the post hoc study on the PLATelet inhibition and patient Outcomes study (PLATO),3 we focused on CAD severity, which, apart from being the best marker for coronary atherosclerosis burden, indirectly reflects the co-morbidity burden. Thus, the study3 found a 1-year risk of 16.3% when having MVD (defined as 3-VD, LMS or prior CABG), and a study6 restricted to health insurance beneficiaries demonstrated an almost 21% risk after 4 years in high-risk (defined as DM, prior MI or/and chronic end stage kidney disease) stable post-MI patients. When focusing on overall estimates, a recent nationwide Swedish study5 reported, similarly to our study, an 18.3% risk on the first year but a higher risk in the stable post-MI population (20% after 3 years vs. 21% after 4 years). This latter difference might reflect the fact that we, as opposed to Jernberg et al.,5 excluded high-risk patients (non-fatal CV events) at baseline. In a Spanish study, the corresponding estimates after 1 and 4 years were 7.3% and 10.1%, respectively.4 These lower estimates might be expected in single-centre studies with higher rate of revascularization and with a closer clinical follow-up program. Nevertheless, we showed a relatively high residual risk and that the risk increased with increasing CAD severity not only during the first year (8.4–26.4%) but also in the successive years (13.6–25.2%).
More importantly, those treated non-invasively had the highest risk (35.5% and 41.0%), as reported elsewhere.7,35,42,43 However, after adjusting for confounders including those related to type 2 MI, MVD appeared as the most important risk factor for cardiovascular events at 1-year and beyond. This is supported by 1-year- and partially by longer follow-up studies.3,4,38 Similar to others,4 we showed that the relative importance of MVD was greater in the first year than in the following years. At the same time, the importance of co-morbidity in relation to MVD was more pronounced in the stable post-MI population, which is in line with other studies.4,38 It is important to bear in mind that some co-morbidities (e.g., cerebrovascular disease and peripheral artery disease) share the same risk factor as for CAD, and in general, the extent of atherosclerosis in the coronary and non-coronary beds is a strong determinant of long-term prognosis.44 This suggests that the impact of factors other than MVD becomes more important during long-term follow-up after the CAD has stabilized.
Follow-up and secondary prevention
We showed a slightly lower degree of initiation of secondary preventive drugs (β-blockers, aspirin P2Y12 inhibitors and statins) and utilization of invasive strategy in MI as compared to other national studies.7,32 The most likely reason for this difference lies in the shorter period for initiating the medication and recording the invasive procedures. Similar to other studies from abroad, the majority received evidence-based therapies for CAD at baseline4,5 and remained on it in the stable post-MI population.5 Although there have been improvements in initiating secondary preventive drugs, a large group of MI patients faced undertreatment (primarily in non-obstructive CAD and conservative strategy) as reported elsewhere45; thus, initiating medication at discharge is a critical factor for medication adherence.46 Performing CAG is another critical factor that plays a part in the initiation of secondary prevention drugs.7,47,48 A significant proportion of both study populations were assigned a conservative strategy, a proportion similar to other studies on MI and stable CAD.32,35,37,42,47,48
With increasing CAD severity, the rate of revascularization dropped and the prevalence of nitrates increased, and for those selected for a conservative strategy, a less aggressive treatment was given. As in previous studies,7,42,43 these patients also appeared to be older and with greatest co-morbidity burden, crucial factors that are usually considered in the risk benefit analysis of an invasive strategy.47,48 While some found conservative strategy as being clinically justifiable,47 others showed that the risk profile has been underestimated,49 indicating a risk-treatment mismatch where high-risk patients have a lower likelihood to receive CAG,40,42 even though the benefit of an invasive strategy increases with baseline risk.50
In this present study, we showed that the residual risk in stable post-MI patients with MVD was increased even though we adjusted for treatment. This indicates that selected patients might benefit from individualized treatment. That said, we also need to be better to achieve our treatment goals, as a large share of patients received suboptimal secondary prevention drugs (more pronounced in stable post-MI), invasive strategy (more pronounced in index MI) and according to the Euroaspire survey III do not enter cardiac rehabilitation programs after discharge.8
Special consideration of myocardial infarction subgroups
Special attention should be made to patients with no significant stenosis and conservatively treated patients, as their underlying cause for MI is different from obstructive CAD.22,31 Both represent the opposite ends of the risk spectrum. As outlined above, the high risk associated with conservative strategy is understandable. Similarly, although the risk is lower in patients with no significant stenosis compared to those with more extensive CAD, it is still high and might be linked to undertreatment as well,41 which is in line with our results. More importantly, for both populations, there is no clear evidence of appropriate treatment recommendations even though these patients are not rare in clinical practice.
Limitations
The strength of this study lies in the large nationwide register-based cohort design, which provided a national study population on MI with close to complete information on CAD severity (8% missing) among CAD recipients and long-term follow-up data. A number of limitations inherent to the nature of retrospective studies are present. First, given that the positive predictive of the diagnosis for MI has been found high,51 it was not possible to differentiate among STEMI, NSTEMI, and type 2 MI, which is a subgroup with emerging interest. Thus, the case mix of MI patients affect the generalizability of the study results, as STEMI and NSTEMI have different risk/prognosis. Second, in 2010, the DHR changed its data structure, and therefore, some variables had missing data, including for the CAD severity for 2010. We believe the information on CAD severity was missing at random. Another important point is that the DHR has national coverage on CAG only from 2006, which meant that a higher proportion of CAG without information on CAD (obtained from the DNPR) was noted in the preceding years. Sensitivity analyses showed no significant changes in the main results, other than an almost 7% point reduction in the overall cumulative incidence noted in both groups when conservatively treated patients were excluded. Third, some patients with LMS had evidence of 2- or 3-VD, giving them a more different risk profile. Furthermore, the estimation of CAD severity relied on the basis of the number of coronary vessels with a stenosis more than 50%, which is somehow less informative than such as the Syntax score, which was not registered in the DHR. Fourth, regardless of the number of CAG performed during index hospitalization, patients with different findings on the angiogram were categorized according to the most severe CAD severity. Fifth, during the study years, the implementation of high-sensitive assays most likely, to a certain extent, resulted in reclassification of unstable angina pectoris to NSTEMI, which meant that certain NSTEMI might have had a lower risk than NSTEMI in the preceding period. Finally, there is a lack of information about important clinical parameters such as complete vs. incomplete revascularization, fibrinolytic agents, left ventricular ejection fraction, infarct size, blood pressure, body mass index, lipid levels, and cardiac rehabilitation programs. Thus, we were unable to account for achievement of treatment goals, and therefore, we cannot rule out the effect of unmeasured confounders while estimating the effect of CAD severity and co-morbidity.
Conclusions
This nationwide study showed that stable post-MI patients had increased risk of cardiovascular events beyond the first year and was strongly related to CAD severity. Despite medical treatment and that a large part of patients with multi-vessel disease underwent revascularization, CAD severity was the strongest risk factor for recurrent cardiovascular event in a long-term perspective.
Supplementary material
Supplementary material is available at European Heart Journal—Cardiovascular Pharmacotherapy online.
Funding
This work was supported by AstraZeneca.
Conflict of interest: A.D. and H.N.C. are employed by AstraZeneca. G.H.G. is supported by an unrestricted research scholarship from the Novo Nordisk Foundation and reports research grants from AstraZeneca, Pfizer, Bayer, Bristol-Myers Squibb, and Boehringer Ingelheim.
Supplementary Material
References
- 1. Schmidt M, Jacobsen JB, Lash TL, Botker HE, Sorensen HT.. 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: a Danish nationwide cohort study. BMJ 2012;344:e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M.. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J 2016;37:3232–3245. [DOI] [PubMed] [Google Scholar]
- 3. Kotsia A, Brilakis ES, Held C, Cannon C, Steg GP, Meier B, Cools F, Claeys MJ, Cornel JH, Aylward P, Lewis BS, Weaver D, Brandrup-Wognsen G, Stevens SR, Himmelmann A, Wallentin L, James SK.. Extent of coronary artery disease and outcomes after ticagrelor administration in patients with an acute coronary syndrome: insights from the PLATelet inhibition and patient Outcomes (PLATO) trial. Am Heart J 2014;168:68–75e2. [DOI] [PubMed] [Google Scholar]
- 4. Abu-Assi E, Lopez-Lopez A, Gonzalez-Salvado V, Redondo-Dieguez A, Pena-Gil C, Bouzas-Cruz N, Raposeiras-Roubín S, Riziq-Yousef Abumuaileq R, García-Acuña JM, González-Juanatey JR.. The risk of cardiovascular events after an acute coronary event remains high, especially during the first year, despite revascularization. Rev Esp Cardiol 2016;69:11–18. [DOI] [PubMed] [Google Scholar]
- 5. Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M.. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J 2015;36:1163–1170. [DOI] [PubMed] [Google Scholar]
- 6. Kern DM, Mellstrom C, Hunt PR, Tunceli O, Wu B, Westergaard M, Hammar. Long-term cardiovascular risk and costs for myocardial infarction survivors in a US commercially insured population. Curr Med Res Opin 2016;32:703–711. [DOI] [PubMed] [Google Scholar]
- 7. Hvelplund A, Galatius S, Madsen M, Sorensen R, Madsen JK, Iversen AZ, Tilsted HH, Helqvist S, Mortensen PE, Nielsen PH, Prescott E, Abildstrøm SZ.. Significance of the invasive strategy after acute myocardial infarction on prognosis and secondary preventive medication: a nationwide study of 6364 women and 11,915 men. J Invasive Cardiol 2012;24:19–24. [PubMed] [Google Scholar]
- 8. Kotseva K, Wood D, De Bacquer D, De Backer G, Ryden L, Jennings C, Kotseva K, Wood D, De Bacquer D, De Backer G, Rydén L, Jennings C, Gyberg V, Amouyel P, Bruthans J, Castro Conde A, Cífková R, Deckers JW, De Sutter J, Dilic M, Dolzhenko M, Erglis A, Fras Z, Gaita D, Gotcheva N, Goudevenos J, Heuschmann P, Laucevicius A, Lehto S, Lovic D, Miličić D, Moore D, Nicolaides E, Oganov R, Pajak A, Pogosova N, Reiner Z, Stagmo M, Störk S, Tokgözoğlu L, Vulic D; EUROASPIRE Investigators. EUROASPIRE IV: a European Society of Cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur J Prev Cardiol 2016;23:636–648. [DOI] [PubMed] [Google Scholar]
- 9. Eyre H, Kahn R, Robertson RM, Clark NG, Doyle C, Hong Y, Gansler T, Glynn T, Smith RA, Taubert K, Thun MJ, American Cancer Society, the American Diabetes Association, and the American Heart Association Collaborative Writing Committee. Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Circulation 2004;109:3244–3255. [DOI] [PubMed] [Google Scholar]
- 10. Parker AB, Naylor CD, Chong A, Alter DA, Socio-Economic Status and Acute Myocardial Infarction Study Group. Clinical prognosis, pre-existing conditions and the use of reperfusion therapy for patients with ST segment elevation acute myocardial infarction. Can J Cardiol 2006;22:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gili M, Sala J, Lopez J, Carrion A, Bejar L, Moreno J, Rosales A, Sánchez G.. [Impact of comorbidities on in-hospital mortality from acute myocardial infarction, 2003-2009]. Rev Esp Cardiol 2011;64:1130–1137. [DOI] [PubMed] [Google Scholar]
- 12. McManus DD, Nguyen HL, Saczynski JS, Tisminetzky M, Bourell P, Goldberg RJ.. Multiple cardiovascular comorbidities and acute myocardial infarction: temporal trends (1990-2007) and impact on death rates at 30 days and 1 year. Clin Epidemiol 2012;4:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen HY, Saczynski JS, McManus DD, Lessard D, Yarzebski J, Lapane KL, Gore JM, Goldberg R.. The impact of cardiac and noncardiac comorbidities on the short-term outcomes of patients hospitalized with acute myocardial infarction: a population-based perspective. Clin Epidemiol 2013;5:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Radovanovic D, Seifert B, Urban P, Eberli FR, Rickli H, Bertel O, Puhan MA, Erne P; AMIS Plus Investigators. Validity of Charlson Comorbidity Index in patients hospitalised with acute coronary syndrome. Insights from the nationwide AMIS Plus registry 2002-2012. Heart 2014;100:288–294. [DOI] [PubMed] [Google Scholar]
- 15. Mock MB, Ringqvist I, Fisher LD, Davis KB, Chaitman BR, Kouchoukos NT, Kaiser GC, Alderman E, Ryan TJ, Russell RO Jr, Mullin S, Fray D, Killip T 3rd. Survival of medically treated patients in the Coronary Artery Surgery Study (CASS) registry. Circulation 1982;66:562–568. [DOI] [PubMed] [Google Scholar]
- 16. Yusuf S, Zucker D, Peduzzi P, Fisher LD, Takaro T, Kennedy JW, Davis K, Killip T, Passamani E, Norris R, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet 1994;344:563–570. [DOI] [PubMed] [Google Scholar]
- 17. Mark DB, Nelson CL, Califf RM, Harrell FE Jr., Lee KL, Jones RH, Fortin DF, Stack RS, Glower DD, Smith LR, et al. Continuing evolution of therapy for coronary artery disease. Initial results from the era of coronary angioplasty. Circulation 1994;89:2015–2025. [DOI] [PubMed] [Google Scholar]
- 18. Ikeno F, Brooks MM, Nakagawa K, Kim MK, Kaneda H, Mitsutake Y, Vlachos HA, Schwartz L, Frye RL, Kelsey SF, Waseda K, Hlatky MA; BARI-D Study Group. SYNTAX score and long-term outcomes: the BARI-2D Trial. J Am Coll Cardiol 2017;69:395–403. [DOI] [PubMed] [Google Scholar]
- 19. Califf RM, Harrell FE Jr, Lee KL, Rankin JS, Hlatky MA, Mark DB, Jones RH, Muhlbaier LH, Oldham HN Jr, Pryor DB.. The evolution of medical and surgical therapy for coronary artery disease. A 15-year perspective. JAMA 1989;261:2077–2086. [PubMed] [Google Scholar]
- 20. Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, Lippolis NJ, Berman DS, Callister TQ.. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol 2007;50:1161–1170. [DOI] [PubMed] [Google Scholar]
- 21. Neeland IJ, Patel RS, Eshtehardi P, Dhawan S, McDaniel MC, Rab ST, Vaccarino V, Zafari AM, Samady H, Quyyumi AA.. Coronary angiographic scoring systems: an evaluation of their equivalence and validity. Am Heart J 2012;164:547–552e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, Baumgartner H, Gaemperli O, Achenbach S, Agewall S, Badimon L, Baigent C, Bueno H, Bugiardini R, Carerj S, Casselman F, Cuisset T, Erol Ç, Fitzsimons D, Halle M, Hamm C, Hildick-Smith D, Huber K, Iliodromitis E, James S, Lewis BS, Lip GY, Piepoli MF, Richter D, Rosemann T, Sechtem U, Steg PG, Vrints C, Luis Zamorano J; Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 23. Schmidt M, Pedersen L, Sorensen HT.. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 2014;29:541–549. [DOI] [PubMed] [Google Scholar]
- 24. Lynge E, Sandegaard JL, Rebolj M.. The Danish National Patient Register. Scand J Public Health 2011;39:30–33. [DOI] [PubMed] [Google Scholar]
- 25. Helweg-Larsen K. The Danish Register of Causes of Death. Scand J Public Health 2011;39:26–29. [DOI] [PubMed] [Google Scholar]
- 26. Ozcan C, Juel K, Flensted Lassen J, von Kappelgaard LM, Mortensen PE, Gislason G.. The Danish Heart Registry. Clin Epidemiol 2016;8:503–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kildemoes HW, Sorensen HT, Hallas J.. The Danish National Prescription Registry. Scand J Public Health 2011;39:38–41. [DOI] [PubMed] [Google Scholar]
- 28. Tu JV, Austin PC, Walld R, Roos L, Agras J, McDonald KM.. Development and validation of the Ontario acute myocardial infarction mortality prediction rules. J Am Coll Cardiol 2001;37:992–997. [DOI] [PubMed] [Google Scholar]
- 29. Baron T, Hambraeus K, Sundström J, Erlinge D, Jernberg T, Lindahl B; TOTAL-AMI study group. Type 2 myocardial infarction in clinical practice. Heart 2015;101:101–106. [DOI] [PubMed] [Google Scholar]
- 30. Saaby L, Poulsen TS, Hosbond S, Larsen TB, Pyndt Diederichsen AC, Hallas J, Thygesen K, Mickley H.. Classification of myocardial infarction: frequency and features of type 2 myocardial infarction. Am J Med 2013;126:789–797. [DOI] [PubMed] [Google Scholar]
- 31. Stein GY, Herscovici G, Korenfeld R, Matetzky S, Gottlieb S, Alon D, Gevrielov-Yusim N, Iakobishvili Z, Fuchs S.. Type-II myocardial infarction – patient characteristics, management and outcomes. PloS One 2014;9:e84285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hvelplund A, Galatius S, Madsen M, Rasmussen JN, Rasmussen S, Madsen JK, Sand NP, Tilsted HH, Thayssen P, Sindby E, Højbjerg S, Abildstrøm SZ.. Women with acute coronary syndrome are less invasively examined and subsequently less treated than men. Eur Heart J 2010;31:684–690. [DOI] [PubMed] [Google Scholar]
- 33. Sandoval Y, Smith SW, Thordsen SE, Apple FS.. Supply/demand type 2 myocardial infarction: should we be paying more attention? J Am Coll Cardiol 2014;63:2079–2087. [DOI] [PubMed] [Google Scholar]
- 34. Widimsky P, Holmes DR Jr.. How to treat patients with ST-elevation acute myocardial infarction and multi-vessel disease? Eur Heart J 2011;32:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Terkelsen CJ, Lassen JF, Nørgaard BL, Gerdes JC, Jensen T, Gøtzsche LB, Nielsen TT, Andersen HR.. Mortality rates in patients with ST-elevation vs. non-ST-elevation acute myocardial infarction: observations from an unselected cohort. Eur Heart J 2005;26:18–26. [DOI] [PubMed] [Google Scholar]
- 36. Mehta SR, Granger CB, Boden WE, Steg PG, Bassand JP, Faxon DP, Afzal R, Chrolavicius S, Jolly SS, Widimsky P, Avezum A, Rupprecht HJ, Zhu J, Col J, Natarajan MK, Horsman C, Fox KA, Yusuf S; TIMACS Investigators. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med 2009;360:2165–2175. [DOI] [PubMed] [Google Scholar]
- 37. Steg PG, Greenlaw N, Tardif JC, Tendera M, Ford I, Kääb S, Abergel H, Fox KM, Ferrari R; CLARIFY Registry Investigators. Women and men with stable coronary artery disease have similar clinical outcomes: insights from the international prospective CLARIFY registry. Eur Heart J 2012;33:2831–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bauters C, Deneve M, Tricot O, Meurice T, Lamblin N, Investigators C. Prognosis of patients with stable coronary artery disease (from the CORONOR study). Am J Cardiol 2014;113:1142–1145.24507170 [Google Scholar]
- 39. Jespersen L, Abildstrom SZ, Hvelplund A, Madsen JK, Galatius S, Pedersen F, Hojberg S, Prescott E.. Burden of hospital admission and repeat angiography in angina pectoris patients with and without coronary artery disease: a registry-based cohort study. PloS One 2014;9:e93170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cohen MG, Filby SJ, Roe MT, Chen AY, Menon V, Stouffer GA, Gibler WB, Smith SC Jr, Pollack CV Jr, Peterson ED, Ohman EM.. The paradoxical use of cardiac catheterization in patients with non-ST-elevation acute coronary syndromes: lessons from the Can Rapid Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines (CRUSADE) Quality Improvement Initiative. Am Heart J 2009;158:263–2670. [DOI] [PubMed] [Google Scholar]
- 41. Pizzi C, Xhyheri B, Costa GM, Faustino M, Flacco ME, Gualano MR, Fragassi G, Grigioni F, Manzoli L.. Nonobstructive versus obstructive coronary artery disease in acute coronary syndrome: a meta-analysis. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Blatt A, Kalmanovich E, Karny-Rahkovich O, Brener S, Shlezinger M, Shlomo N, Vered Z, Hod H, Goldenberg I, Elbaz-Greener G.. Comparison of noninvasively and invasively managed patients, with or without revascularization in non-ST elevation myocardial infarction (from the Acute Coronary Syndrome Israeli Survey). Am J Cardiol 2016;118:1–5. [DOI] [PubMed] [Google Scholar]
- 43. Libungan B, Karlsson T, Albertsson P, Herlitz J.. Elderly patients with myocardial infarction selected for conservative or invasive treatment strategy. Clin Interv Aging 2015;10:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, Wilson PW, Alberts MJ, D'Agostino R, Liau CS, Mas JL, Röther J, Smith SC Jr, Salette G, Contant CF, Massaro JM, Steg PG; REACH Registry Investigators. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA 2010;304:1350–1357. [DOI] [PubMed] [Google Scholar]
- 45. Jorgensen CH, Gislason GH, Ahlehoff O, Andersson C, Torp-Pedersen C, Hansen PR.. Use of secondary prevention pharmacotherapy after first myocardial infarction in patients with diabetes mellitus. BMC Cardiovasc Disord 2014;14:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gislason GH, Rasmussen JN, Abildstrøm SZ, Gadsbøll N, Buch P, Friberg J, Rasmussen S, Køber L, Stender S, Madsen M, Torp-Pedersen C.. Long-term compliance with beta-blockers, angiotensin-converting enzyme inhibitors, and statins after acute myocardial infarction. Eur Heart J 2006;27:1153–1158. [DOI] [PubMed] [Google Scholar]
- 47. Olivari Z, Chinaglia A, Gonzini L, Falsini G, Pilleri A, Valente S, Gregori G, Rollo R, My L, Scrimieri P, Lanzillo T, Corrado L, Chiti M, Picardi E; on behalf of BLITZ 4 Investigators. Invasive strategy in non-ST-segment elevation acute coronary syndrome: what should be the benchmark target in the real world patients? Insights from BLITZ-4 Quality Campaign. Int J Cardiol 2016;220:761–767. [DOI] [PubMed] [Google Scholar]
- 48. Amsterdam EA, Peterson ED, Ou FS, Newby LK, Pollack CV Jr, Gibler WB, Ohman EM, Roe MT.. Comparative trends in guidelines adherence among patients with non-ST-segment elevation acute coronary syndromes treated with invasive versus conservative management strategies: results from the CRUSADE quality improvement initiative. Am Heart J 2009;158:748–754e1. [DOI] [PubMed] [Google Scholar]
- 49. Lee CH, Tan M, Yan AT, Yan RT, Fitchett D, Grima EA, Langer A, Goodman SG; Canadian Acute Coronary Syndromes (ACS) Registry II Investigators. Use of cardiac catheterization for non-ST-segment elevation acute coronary syndromes according to initial risk: reasons why physicians choose not to refer their patients. Arch Intern Med 2008;168:291–296. [DOI] [PubMed] [Google Scholar]
- 50. Fox KA, Clayton TC, Damman P, Pocock SJ, de Winter RJ, Tijssen JG, Lagerqvist B, Wallentin L; FIR Collaboration. Long-term outcome of a routine versus selective invasive strategy in patients with non-ST-segment elevation acute coronary syndrome a meta-analysis of individual patient data. J Am Coll Cardiol 2010;55:2435–2445. [DOI] [PubMed] [Google Scholar]
- 51. Coloma PM, Valkhoff VE, Mazzaglia G, Nielsson MS, Pedersen L, Molokhia M, Mosseveld M, Morabito P, Schuemie MJ, van der Lei J, Sturkenboom M, Trifirò G; EU-ADR Consortium. Identification of acute myocardial infarction from electronic health-care records using different disease coding systems: a validation study in three European countries. BMJ Open 2013;3:e002862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.